Abstract
In this paper, specific conditions for palladium as well as silver deposition were determined. Also, the simultaneous, co-deposition of the same was determined for hydrazine-based solution bearing both element precursors. The electroless plating kinetics for simultaneous, co-depositions for both elements in the mixture were determined. Moreover, a mathematical model to determine the membrane thickness, the rate of plating and, composition of profile with respect to plating parameters were developed as well as the structural evolution in the course of plating was also investigated (Cheng and Yeung, 127). As such, a hydrogen permeation study revealed that given common thickness, a composite membrane of Pd/Ag dwarfs pure palladium membrane in performance. Additionally, with all the factors constant, the performance of the membrane is invertedly proportional to the membranes’ thickness.
Focus of the paper
In a synopsis, this paper focuses on a glimpse of the background information about flexible thin membranes, eventually narrowing down to the process of manufacture of Palladium-silver composite membrane in hydrazine-based plating bath thanks to electroless plating technique. In particular, this paper highlights on the plating kinetics, film quality and efficiency notwithstanding. Moreover, the paper focuses on the gross evolution and development of the microstructure in the course of plating courtesy of X-ray photoelectron spectroscopy and X-ray diffraction. Conclusively, the study underscores the relationship between alloy formation and permeation efficiency (Tsuji 78).
Introduction
Thin film layer, as the name suggests, is a very slim layer of some material which thickness is measured in fractions as it can be from few nanometers (monolayer) to several micrometers. To this end, this technology finds its main applications in electronic semiconductor devices as well as in optical devices as optical coatings. A common example of a thin film application in our homes is a mirror which has a thin silvery coating that acts as a reflecting surface.
To improve the effectiveness of optical coatings, such as in antireflective otherwise AR coatings, multiple layers with different thicknesses as well as refractive indices are embedded as one. Akin to this technology, intervallic structure of intermittent thin films results in a material known as superlattice which limits electronic experience to two-dimension thanks to quantum confinement phenomenon. To date, advancement in technology is carried out on ferroelectric and ferromagnetic thin films to find applications in computer memory. In pharmaceutical field, thin film technology finds application courtesy of thin film drug delivery. Notably, thin film technology is used in the production of thin-film batteries as well as dye-sensitized solar cells (Tsuji 79).
The actual process of application of a thin film on a substrate is referred to as thin-film deposition. Basically, the term ‘Thin’ is relative depending on the technique used in processing of a given layer (Williams and Carter, 11). Generally, most of these techniques vary their monolayer thickness within a bracket of a few folds of tens of a nanometer. Deposition of a monolayer of atoms is usually achieved courtesy of a molecular beam epitaxy which also finds application in the manufacture of packaging materials e.g. aluminum-coated PET films. Similar processes are “sometimes used where thickness is not important: for instance, the purification of copper by electroplating” (Crabtree 100). As such, deposition techniques can broadly be grouped into classes; physical and chemical deposition.
In view of the chemical deposition technique, a fluid precursor is transformed on contact with a solid surface, resulting in the formation of a thin layer. Since the liquid encloses the entire solid substrate, deposition occurs absolutely on the whole surface. Furthermore, depending on the precursor phases, this technique can further be categorized into five classes including: Planting, Chemical solution deposition (CSD), Spin coating, chemical vapor deposition (CVD) and, Atomic layer deposition (ALD). Specifically, while Planting relies on a solution of water and a salt of a metal as precursor, CSD relies on an organometallic powder in an organic solvent for the same (Vorotyntsev, Zinovyeva and Konev, 28). On the other hand, Spin coating relies on sol-gel precursor while CVD uses gas-phase precursor usually a halide. ALD uses gaseous precursors to achieve a thin layer (Crabtree 107).
In light of the physical deposition technique, a monolayer film of solid is deposited mechanically, thermodynamically or, electromechanically. To this end it is reputed that just like engineering materials which are held intact by relatively high energies, and with devoid of chemical energies, these techniques perform efficiently under low-pressure vapor environments. As such, it has been christened physical vapor deposition technique (PVD). Normally, the precursor is confined within an energetic, entropic niche, for the purpose of enhancing the atoms to leave its surface. On the recipient end a cooler surface absorbs the energy from the deposited atoms as they for a solid thin layer. The process is holistically done in a vacuum to achieve the best results. Basically, physical deposition relies on a thermal evaporator with molecular beam epitaxy (MBE), a more sophisticated type and the most efficient. A thermal evaporator “uses an electric resistance heater to melt material and raise its vapor pressures to useful range” (Morton 123). Other examples include: Electron beam evaporator, Sputtering, Pulsed laser deposition, Cathodic arc deposition (arc-PVD) and Electrohydrodynamic deposition.
With regard to this paper, we take a glimpse at the process of manufacture of Palladium-silver composite membrane thanks to electroless plating technique. Palladium-silver (Pd/Ag) membrane is vital in the production of environmental friendly fuel. Faced with quite a massive deposit of crude oil and, with the environmentalists’ outcry on zero-emission vehicles, the demand for hydrogen as an element for fuel purification is projected to skyrocket. To this end, production processes with regard to fuel products need to be advanced to enhance the effective utilization of hydrogen. Hydrogen selective membranes form the better part of intensive research in recent years (Cheng and Yeung, 127). Apart from its use as a fuel purifier, Pd/Ag membrane is useful in hydrogen elimination in process streams such as in dehydrogenation processes. With regard to the performance, palladium membranes are known for their higher selectivity degree (Cheng and Yeung, 128). Significantly, thin film palladium membranes enhance higher permeation flux.
Electroless plating aside, palladium membranes are also prepared using sputtering. CVD is later used to enhance the selective ability of this membrane. However, sputter coating has relatively low efficiency and is costly in preparation. In regard to membrane purity, pure palladium membranes are known to suffer from hydrogen embrittleness and are as well sensitive to trace elements (e.g. organohalides) which are present in process streams. This makes palladium composite membranes favorable in entirely all processes.
Specifically, this research focuses on the preparation of Pd/Ag membrane using the electroless plating method in a hydrazine-based plating bath. Considering the plating kinetics, film quality and plating efficiency are also studied. Also, a gross evolution of its microstructure is also analyzed courtesy of X-ray photoelectron spectroscopy and X-ray diffraction. As a result, the study reveals a correlation between allow development and permeation efficiency.
Experimental procedure
Palladium and silver co-deposition
In this procedure, the co-deposition of palladium and silver elements into solid thin layer within hydrazine-based solution was researched. The plating solution was composed of “EDTA stabilized Pd-amine and Ag-amine metal complexes with hydrazine as a reducing agent” (Cheng and Yeung, 128). To stabilize plating bath as well as the PH, excess ammonium hydroxide was used (Cheng and Yeung, 128). Vitally, care was taken to maintain the appropriated conditions for simultaneous, co-deposition lest consecutive deposition occur that would rather result to a bi-layer as opposed to the expected monolayer. The temperature conditions were maintained at 1K. A stimulated porous vycor tube of an area of about 5 cm2 was then placed in the solution for a period of 1 hour. For the sake of structural analysis, the tube was cut along its cross-section for easy viewing.
The structure was observed using an SME, JEOL JSM-6300 scanner. The thickness, plating rates and characterization were also done. Moreover, the composition of the film layer was also determined.
Regarding the electroless plating kinetics, the amount of the substrate deposited was later on monitored over time to an accuracy of 0.01µg/cm2. The concentrations were also varied systematically and growth monitored at a constant time of 10 minutes. Finally, with respect to alloy formation and membrane permeation, the gas permeation flow rates were later on determined using a bubble flow-meter and this was done at room temperature and pressure (Cheng and Yeung, 130).
Results/Analysis
A correlation was done for the plating parameters with respect to membrane permeation rates as well as film microstructure for the hydrazine-based palladium-silver plating solution.
In regard to palladium-silver co-deposition, the results below were obtained.
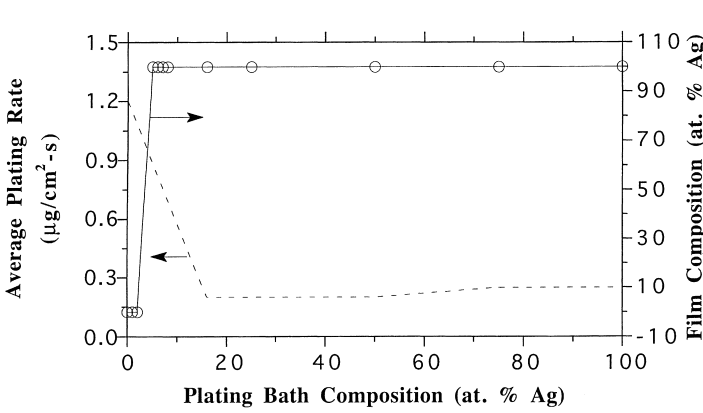
For the investigation of plating conditions, the following graphs were obtained:

Discussion and Conclusion
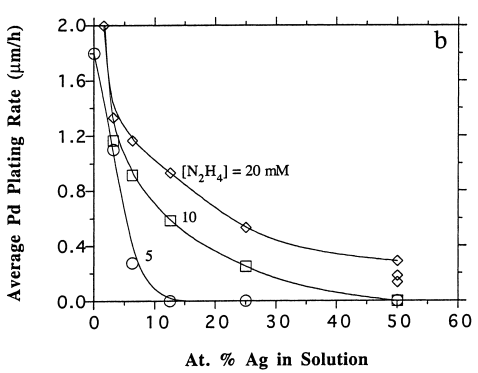
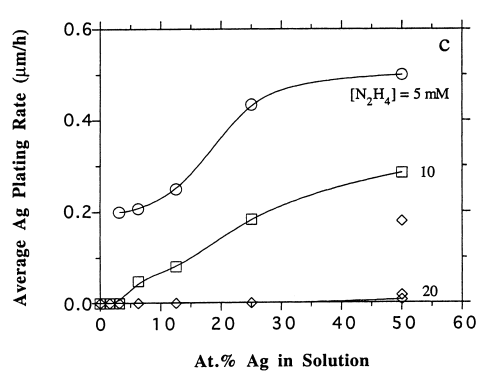
Figure 1 above shows a relationship between palladium and silver precursors co-deposition behaviors in hydrazine-based plating solution. The results as portrayed in the figure indicate that the degree of concentration of silver elements in the solution limits the deposition of palladium precursors. Consequently, this limits the overall plating efficiency of the metal precursors. A correlation between silver content in the membrane and its concentration in the plating solution indicates that even at lower concentrations of at most 5%, pure silver films are achievable. The figure also exhibits the average plating efficiency as having a low at about 40% Ag and has individual element’s deposition rates higher than for the mixture (Cheng and Yeung, 131).
The reason for the above behavior has a bearing on the type of reaction that takes place among the three elements (hydrazine, Pd-amine, Ag-amine) in the plating bath solution. Basically, oxidation-reduction reaction is the reason behind the behavior. Hydrazine acts a reducer and as such it favors silver precursors as opposed to palladium consequently resulting in the initial deposition of the former limiting palladium deposition. This results in the formation of pure Ag-film as exhibited above. However, this can be reduced by initially seeding of palladium precursor in the solution or allowing the process to continue for a reasonably longer time (3 hrs.).
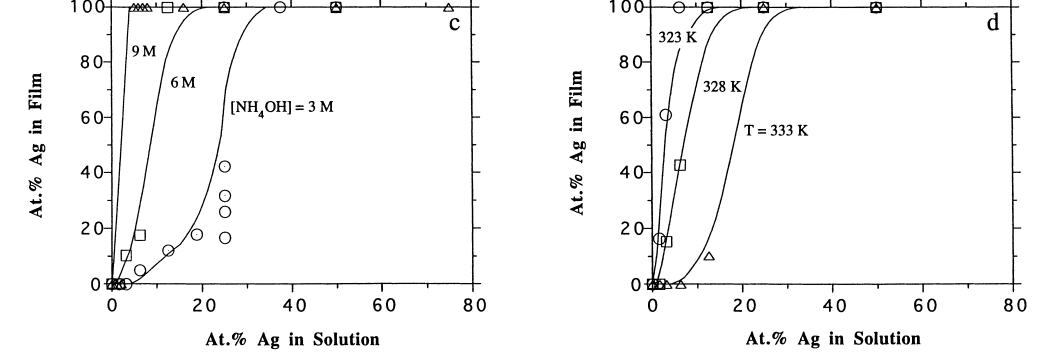
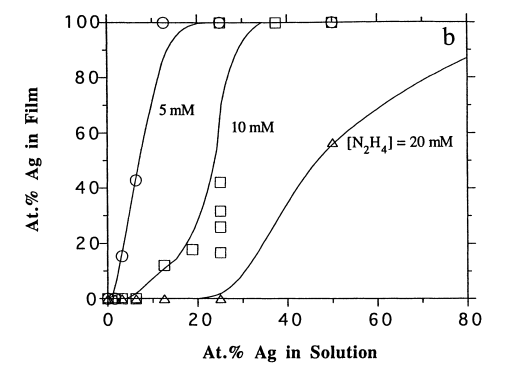
In regard to plating conditions, figure 2 (a) shows the effect of hydrazine concentration on the development Pd/Ag film. Basically, the graph portrays that at higher hydrazine to metal ratio one is in a better position to achieve pure palladium films even at higher Ag concentrations. At lower ammonium hydroxide concentrations (fig. 2 (b)) one is better placed to achieving more palladium deposition. As depicted by graph 2 (c) above more palladium concentration is achievable at higher temperatures as opposed to lower temperatures.
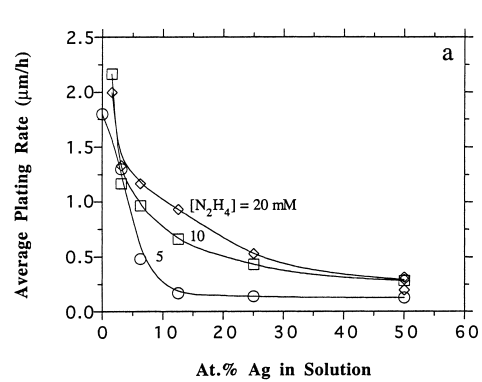
In view of the plating rates, the parameters were measured based on the membrane thickness thanks to electron micrograph scanning (Cheng and Yeung, 132). Both the average and the individual plating rates are shown in figures 3 (a, b and c) above. As demonstrated by graph 3 (a) above, the average plating rate went low with an increase in silver concentration in the hydrazine solution. However, the plating rate raised when hydrazine concentrations increased. The significant decrease in palladium plating with a negligible increase in silver precursor concentration as shown in figure 3 (b) confirms the fact that silver inhibits palladium deposition. Finally, figures 3 (c and b) demonstrates that as opposed to silver plating, increasing hydrazine concentration has a positive impact on the palladium plating. As such, as demonstrated in the figure above (3 (c)) at 20mM hydrazine concentration there is absolutely no Ag plating.
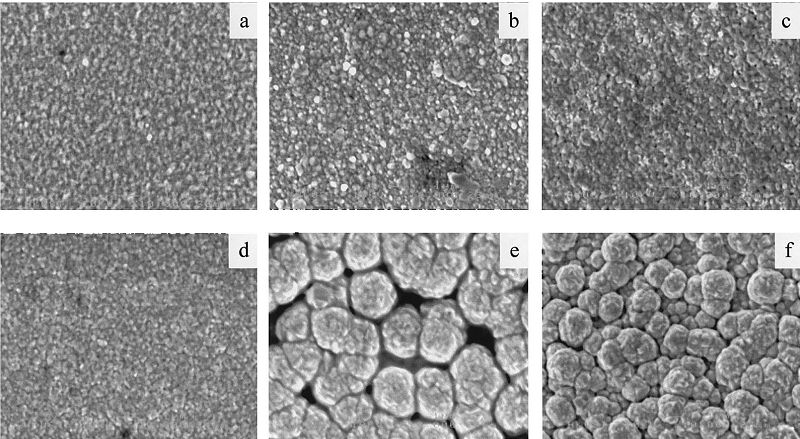
As for the microstructure analysis, figure 4 above demonstrates the different structural architecture of the film under different concentrations of silver with the temperature conditions and hydrazine concentration held constant. As such, figures 4 (a to d) show pictures of microstructure membranes having silver content of 100, 30, 12.5 and 5.5% resulting from plating solutions having silver concentrations of 50, 25, 12.5 and 6.2 respectively. These membranes exhibited continuous film with homogeneous grain size and they portrayed similar microstructure. In the contrary, membranes obtained from silver concentrations of 3.1 and 1.6% (4 (e and d)) had negligible silver content (0-1%), and they resulted in inconsistent microstructure. On drawing comparison between pure palladium microstructure membrane and Pd-Ag membrane it can be seen from the pictures that apart from inhibiting palladium plating, silver does also influence the structural architecture of the film.
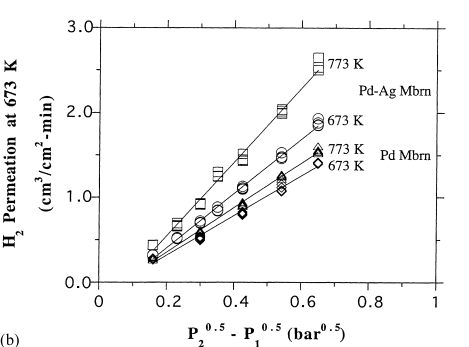
In view of the membrane permeation efficiency (fig. 6) it is evident that for a common thickness the rate of hydrogen permeation for pure palladium membrane is dwarfed by Pd-Ag membrane. Pd-Ag membrane is superior by an order of between 1.4-1.7 times that of pure palladium membrane (Cheng and Yeung, 133).
Conclusion
In a conclusion, in this paper a glimpse into the knowledge about the science behind thin membranes was revisited. Eventually the paper narrowed down to the processing of Pd-Ag membrane where: a synopsis of the optimum conditions for simultaneous co-deposition of individual precursors in Pd-Ag membrane formation was determined. Moreover, the permeation efficiency in regard to membrane composition was also determined and to this end composite membrane outperformed pure membrane.
References
Cheng, Yi-Sheng and King Lun Yeung. “Palladium–silver composite membranes by electroless plating technique”. Journal of Membrane Science. 158.1 (1999), 127-141. Elsevier. Web.
Crabtree, Robert. The Organometallic Chemistry of the Transition Metals. Hoboken, New Jersey: John Wiley and Sons, 2009. Print.
Hou, Kei and Rowling Hughes. “Preparation of thin and highly stable Pd/Ag composite membranes and simulative analysis of transfer resistance for hydrogen separation.” Journal of Membrane Science. 214.1 (2003): 43-44. Print.
Morton, Linda. Palladium sorbents remove contaminants from syngas, DOE Pulse. Denver: MacMurray, 1999. Print.
Tsuji, Jiro. Palladium reagents and catalysts: new perspectives for the 21st century. Hoboken, New Jersey: John Wiley and Sons, 2004. Print.
Vorotyntsev, Mikhail A., Veronica A. Zinovyeva and Dmitry V. Konev. “Mechanisms of Electropolymerization and Redox Activity: Fundamental Aspects.” Electropolymerization. Eds. Serge Cosnier and Arkady Karyakin. Germany: John Wiley & Sons, 2011. 27-47 Print
Williams, David B. and C. Barry Carter. Transmission Electron Microscopy: A Textbook for Materials Science. 2nd ed. New York: Springer, 2009. Print.