Abstract
The development of a continuous monitoring program of the radioisotopes levels has an extreme significance in maintaining the radiological safety of the environment. This study aimed to evaluate the radiological safety of locally-sourced desalinated water and seafood. The establishment of baseline data for observing the nuclear activities already existing in the Gulf region was also targeted. While Cesium radioisotopes serve as the most important indicator of the radiological level, natural Uranium radioisotopes present a particular concern in Kuwait due to the Depleted Uranium (DU) presence after the Gulf War in 1990. The determination of concentrations of Cesium-137 (137Cs), Uranium-234 (234U), and Uranium-238 (238U) has been carried out in Kuwaiti marine water samples through radiochemical analysis and isotopic measurements.
137Cs concentration in the seawater samples was determined by the Ammonium Molybdenum Phosphate (AMP) co-precipitation method followed by gamma spectrometry measurement. 234U and 238U concentrations were determined by a radiochemical separation using anion exchange chromatography and alpha spectrometry measurement. The levels of 137Cs, 234U, and 238U are considered low and analogous to other provincial marine water levels. 134Cs and 235U were below the Minimum Detectable Activity (MDA), which means that these isotopes do not pose any radiological hazard. The produced radiological data can be regarded as the beneficial baseline reference for future analyses in this field. Some Gulf countries, such as the United Arab Emirates (UAE), Qatar, and Saudi Arabia, would initiate their first nuclear reactors within the next few years.
Introduction
The continuous monitoring of radionuclides in marine water is a global demand for maintaining the radiological safety of the nearby areas. The periodic measurement of the environmental radioactivity is essential in detecting any variability in the exposure levels from the natural or anthropogenic sources that may cause an elevated radiation dose. The Arabian Gulf deemed to be among the most polluted marine environments around the world. Approximately one-third of the global oil is exported through the Strait of Hormuz (Al-Zamel et al. 2005). Since this sea passage is directly related to the key source of desalinated water in Kuwait, the verification of its radiological safety is significant in terms of economy and the nation’s health. In addition, the determination of the concentration of Uranium in seawater is extremely important for Kuwait marine to ensure the absence of the DU resulted from weapons used during the Gulf War in 1990.
At present, almost every country in the Gulf area has either a civil nuclear program in progress or a project under consideration, which are expected to supply energy. In particular, the Islamic Republic of Iran launched its Bushehr operational nuclear power plant (NPP) in 2011. The pre-operational nuclear power plants and associated radiological surveys were conducted in the UAE and the Kingdom of Saudia Arabia (KSA). Barakah NPP in the UAE will be operational by 2020 after some delay, and two nuclear reactors will be constructed by 2020 in KSA, which will increase to sixteen units by 2030. A number of environmental and research programs should be completed before the initiation and introduction of these nuclear power plants (Lebanese Atomic Energy Commission – National Council for Scientific Research (CNRS) 2010). Therefore, establishing the baseline radioactivity levels of 137Cs in seawater can be utilized as an indicator of observing elevated levels caused by the nuclear activities in the region.
Uranium natural radioisotopes exist in the earth’s crust and the seawater with a rather long half-life of 246,000 for 234U and 4.47 billion years for 238U. Uranium is classified as a high toxic threat to the public health if humans are exposed to it through ingestion (Carter and Burns 1993). Different analytical methods have been applied for the detection of uranium, including Laser Induced Fluorescence Spectrometry (LIFS), Inductively Coupled Plasma (ICP) emission and mass spectrometry, and thermal ionization mass spectrometry (Huang et al. 2016; UNSCEAR 2000). 137Cs is a common fission product in nuclear reactors regarded as a slightly toxic element decaying by gamma, which can pose a significant and persistent threat due to its intermediate half-life of 30 years, while 134Cs is a nuclear activation product with a half-life of only two years.
Cesium is an electropositive alkaline metal that is capable of contaminating large volumes of water following nuclear accidents. NIH (2005) reports that the major sources of 137Cs and 134Cs were Chernobyl disaster in the 1980s, the nuclear test bombs in the North Hemisphere in the 1960s, and solid waste dumping at sea. There are various methods to identify Cesium in seawater, of which Cesium adsorption of Ammonium Molybdenum Phosphate (AMP) and co-precipitation with various insoluble Hexacyanoferrates (II) present the most widely used ones (Kamenik et al. 2013). The adsorption methods are associated with great promise to timely reveal contamination near nuclear facilities.
A reported incessant 137Cs depositional fluxes were frequently observed in dust fallout in spring and northwestern, southwestern wind periods in Kuwait. The presence of 137Cs in Kuwait maritime area is primarily conditioned by the global atmospheric fallout and the long-range transportation of mainly northwest dust origins (Aba et al. 2018; Biegalski et al. 2001). Consistent with a similar situation in many countries, the nuclear bomb-test experiments in the North Hemisphere as well as Chernobyl disaster specify 137Cs deposition. The greatest amounts of atmospheric fallout were noticed mostly in Europe, Turkey, Syria, and Iraq. As a result, 137Cs was deposited in the form of dust particles in Kuwait Bay through the northwesterly wind. Aba et al. (2014) state that two distinct peaks related to both nuclear events of 137Cs have been observed in the sediment profile of Kuwait.
The regional Gulf levels of 137Cs concentrations were assessed previously in Kuwait and Qatar. 137Cs was 1.05 ± 0.01 mBq L-1 in 2012 and 1.34 ± 0.16 mBq L-1 in 2015 in Kuwait marine water (Uddin et al. 2015), and the total average activity in Qatar marine water was 1.59 ± 0.38 Bq/m3 in both years of 2011 and 2012 (Al-Qaradawi et al. 2015). 238U was 0.040 ± 0.005 mBq L-1, and 234U was 0.054 ± 0.006 mBq L-1 in one location of Kuwait Bay (Uddin et al. 2012). The relevance of this study is related to the shortage in the available radiological data of radionuclide levels in the local seawater.
Study Area
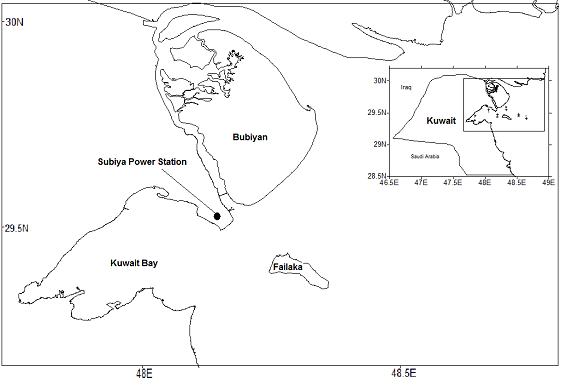
This study involves the Kuwaiti Bay marine water, a partial small bay of the Arabian Gulf situated in the eastern side of the country. Figure 1 shows the sampling area in Kuwait Bay presented in the study. The study area lies at the northwest corner of the Arabian Gulf (AG), which forms the Gulf of Kuwait, locally known as the Jhon, and the area nearby Bobiyan and Failaka islands. The Jhon covers an area of 750 km2, and the water depth varies between 3 and 10 meters with an average depth of about 5 meters (Al-Shemmari et al. 2002). It hosts the major port, namely, Al-Shuwaikh Port and three power and desalination plants: Doha east, Doha west, and Subiya. The Jhon and the adjacent area are characterized by a shallow water throughput with sediment movements by intertidal mudflats. Al-Shamal, the dominant northwesterly wind, severely impacts the Northern area of the AG, shaping the regional dust sources (Khalaf and Al-Hashash 1983). However, the dry land of the southern marshes in Iraq is considered to be a significant source of dust that founders over the northern part of the AG (Al-Ghadban et al. 1999).
Materials and Method
Sampling and Sample Preparation
A cruise was utilized to collect ten high volume marine water samples, 60 liters each, at 1 meter depth from the offshore surface of the Kuwaiti Bay. The samples were collected by pumping seawater into labeled plastic containers. The preservation of samples was ensured by acidifying them with concentrated Hydrochloric acid and storage in plastic containers for further processing in the laboratory.
Sample Analysis
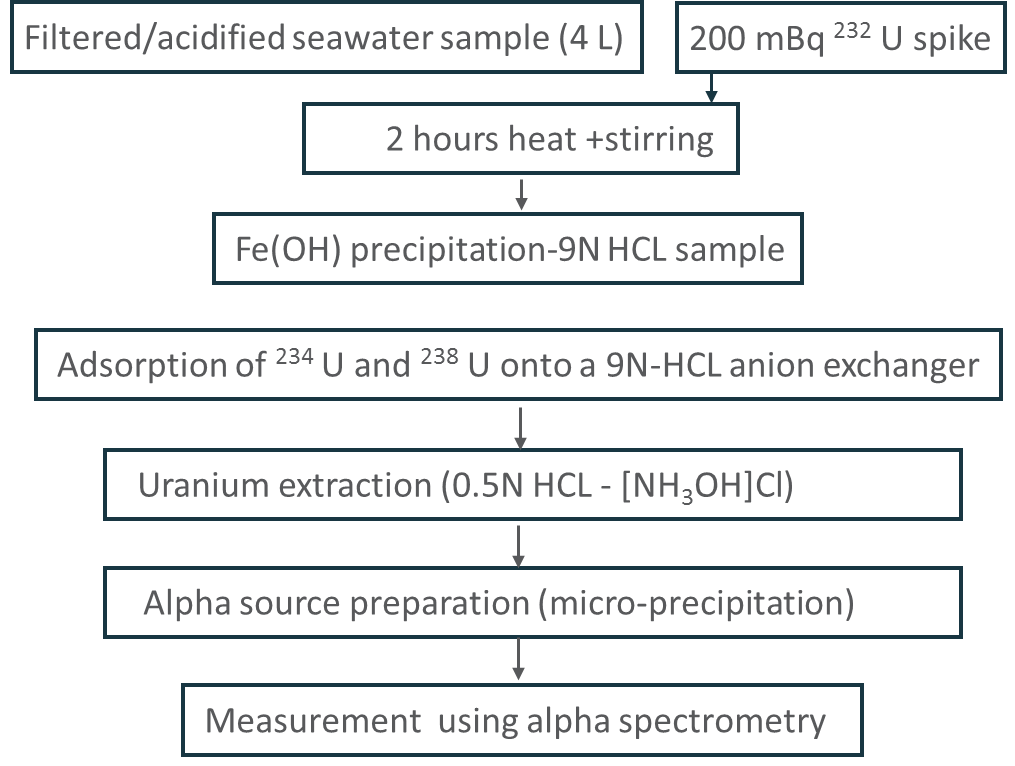
The determination of234U and 238U by a radiochemistry method includes the adsorption of uranium onto 9-N Hydrochloric acid anion exchanger. The given amount of 232U (200 mBq) is added as a radiotracer at the beginning of mineralization. Uranium is directly extracted from resin by eluting 0.5-N Hydrochloric acid- Hydroxyl Ammonium Chloride solution (Yusof et al. 2017). Alpha sample sources are prepared by NdF3 co-precipitation method using 0.1-micrometer propylene 25 mm diameter resolve filters. The prepared alpha sources are counted using alpha spectrometry. Figure 2 demonstrates the procedure followed for Uranium analysis.
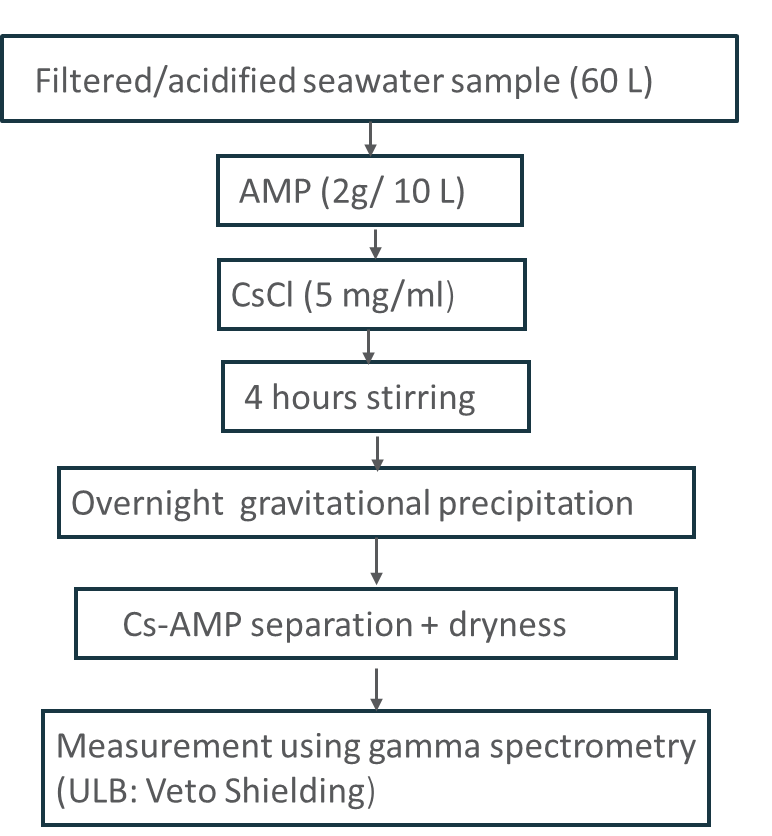
The 137Cs analysis is approached by the adsorption of Cesium into Ammonium Molybdenum Phosphate (AMP) (2 g/10L) and adding some amounts of stable Cesium as a carrier, Cesium Chloride (CsCl), (10 ml of 25 mg/ml solution. Cs- AMP is separated from the sample by gravitational settling and centrifuge of four hours; and, then, the precipitation is collected, dried, and weighted followed by Ultra Low Background gamma spectrometry measurement (Bajoga et al. 2015; Dovelte and Povince 2004). Figure 3 shows the procedure used for Cesium analysis.
Instrumentation and Measurements
The prepared samples are measured by Alpha spectrometry system (Apex, Canberra) equipped with 450 mm2 Passive Implanted Planar Silicon (PIPS) detectors. The detector resolution is about 20–24 keV at 4586 keV, and the detection efficiency at 5 mm (2nd shelf) is about 15 percent. The testers were assessed for a long time (2 days) in order to obtain good calculating statistics. The Ultra-Low Background gamma spectrometry system supplied with High Purity germanium detector and Broad Energy type were employed to determine the radioactivity of 137Cs. The system is linked to an anti-cosmic active Veto shielding to reduce the background by increasing signal to noise ratio, thus revealing very low level concentrations of 137Cs. A standard solution containing a known amount of 137Cs radionuclide was similarly prepared for efficiency calibration. The precipitate was filled in a small petri-dish – 5 cm in diameter. The prepared samples were counted for three days to reach good statistics area at 661.6 keV (uncertainty less than 10 percent). The gamma spectra were analyzed by Genie 2000 software (Canberra Inc.) after background subtraction, and the calculated detection limit was approximately 0.3 mBq L-1.
Activity Concentrations and Chemical Recovery Determination
A blank sample was prepared with the same co-precipitation and separation methods for each radionuclide. This step was critical in considering the background contribution in the measured samples. The blank samples of each radionuclide were used as the background and subtracted from the sample spectra. The specific activity concentration of gamma radionuclide 137Cs was calculated using equation (1) (Dovelt et al. 2004).
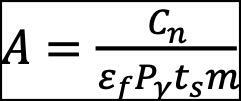
Where A is the activity concentration of a radionuclide in Bq unit;
Cn is net count (background subtracted) of the corresponding full energy peak areas;
εf is the full energy peak detection efficiency;
Pϒ is the emission probability per decay corresponding to the specific gamma-ray energy;
ts is the counting time in term of seconds and m is the mass of water samples in liter (L). Specific activity concentration of alpha radionuclides, 234U and 238U, were calculated using equation (2) (IAEA 2009):
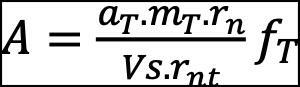
Where A is the activity concentration of a natural Uranium radionuclide in Bq L-1 unit,
at is the specific activity of a tracer (Bq g-1),
mT is the mass of the tracer (g),
rn is the net count rate of the natural radionuclide (background subtracted),
VS is the volume of the sample (L),
rnt is the net count rate of the tracer (background subtracted), and fT is the decay correction factor of a tracer between calibration date and measurement date. The decay of 234U and 238U between separation date and measurement date is negligible because of the long half-lives of the two radioisotopes. The associated uncertainty (u) of the activity concentration of radionuclides was calculated using the error propagation equation (3):
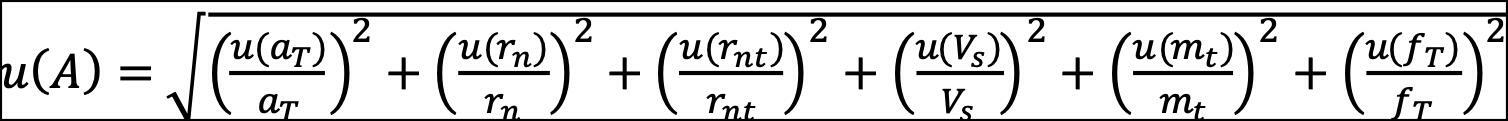
The Minimum Detectable Activity (MDA) was calculated by using Curri formula:
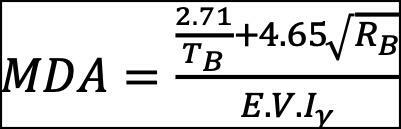
Where TB is the time of background counting,
RB is the count rate of spectroscopy background, E is the detector counting efficiency (%), V is the sample volume,
Iγ is the gamma emission intensity (%). Chemical Recovery (CR %) was calculated for both analytical procedures to ensure the minimal loss of tracer/carrier activity through the analysis.
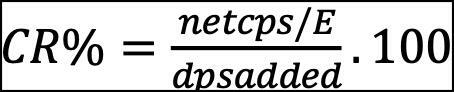

Equation (5) was applied in calculating 232U tracer chemical recovery percentage. Where net cps is the net count per seconds in the spectrum at peak of 5320 keV, dps is the disintegration per second of the added tracer activity, and E is the detection efficiency of the spectrometry (Fadzilah Yousf et al. 2016). CR% of Uranium analysis was 95-100% indicting that most of the Uranium was excreted from the sample. Equation (6) was applied in calculating Cesium-AMP chemical recovery percentage. A repeated co-precipitation was carried out to confirm the completion of 137Cs scavenging from the large seawater samples, and the sample was measured to indicate any remaining 137Cs. The test showed that almost 100% of 137Cs was removed from the sample solution.
Quality Assurance
The certified reference material prepared by the International Atomic Energy Agency, which is coded as IAEA-Irish Sea water-443 containing known amounts of Uranium and 137Cs, was repeatedly analyzed along with the samples to maintain the quality of the analytical results and check the performance of the spectrometries. The obtained results of reference material analysis were in an analogous agreement with the certified values.
Results and Discussion
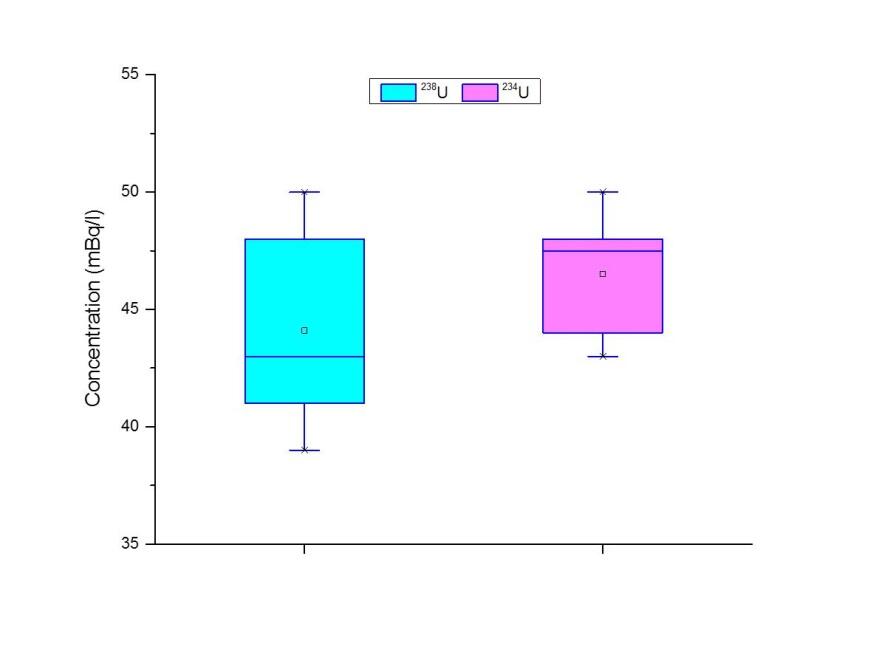
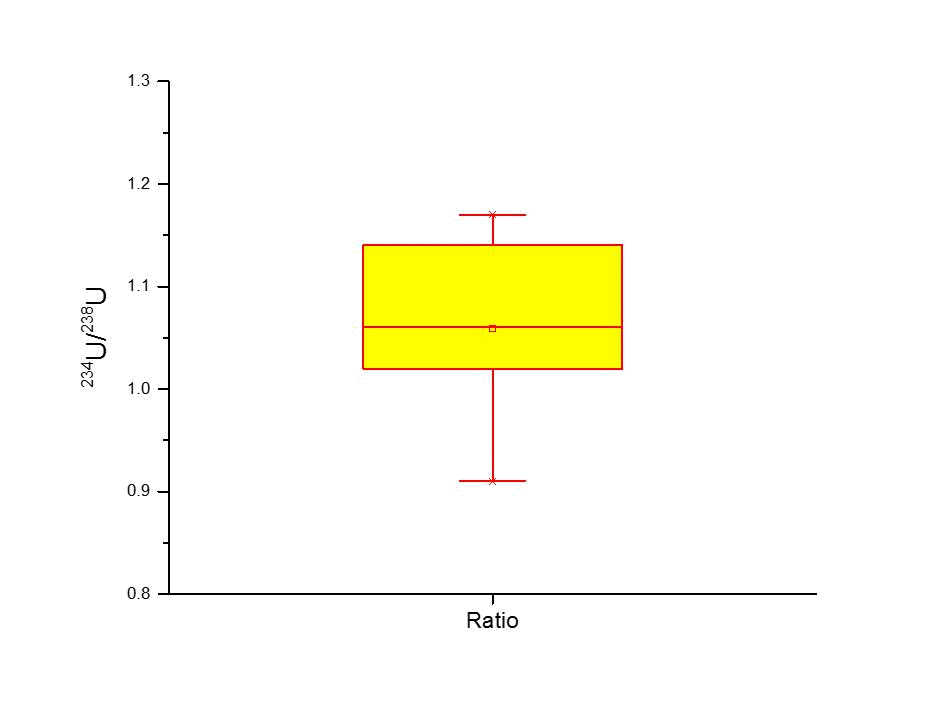
The obtained results of 234U and 238U concentrations are presented in Figure 4. The activity concentrations of 234U and 238U varied from 39 to 50 mBq L-1 with an average value of 45.3 mBq L-1. These values are comparable to the reported Uranium average content of the worldwide seawater (42 mBq L-1 238U) (Monty 2001). It is critical to emphasize that the range of concentrations of Uranium in eastern Mediterranean seawater were 44 to 68 mBq L-1 and 36.9 to 44.5 mBq L-1 in Lebanon and Syria respectively (IAEA 2010; Mamish et al. 2015). The calculated isotopic ratio of 234U/238U in Kuwait Bay water was 1.07 ± 0.01, indicating that there is no anthropogenic source other than the natural provided, which means that the impact of DU is negligible. However, due to the radionuclide recoil process during alpha decay of 238U, the obtained ratio was slightly higher than a unity (Chabaux et al. 2001). The 234U/238U isotopic ratio is consistent with the 234U/238U ratio found in Western North Pacific (1.09 ± 0.05) (Miyake et al. 1966). Conclusively, the near unity value of the 234U/238U ratio in Kuwait Bay seawater specifies the radioactive equilibrium of Uranium and excludes DU resulted from any anthropogenic Uranium sources in Kuwait Bay, DU isotopic ratio is 0.185, whereas natural ratio is 0.935 (WHO 2001). Uranium calculated average isotopic ratio 234U/238U is shown in Figure 5.
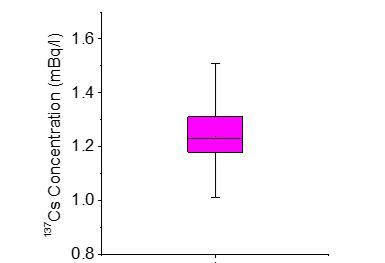
137Cs radioactivity varied between 1.0 and 1.51 mBq L-1 with an average value of 1.24 mBq L-1, as exemplified in Figure 6. This range of 137Cs concentration is comparable to that reported in the Gulf (1.6 ± 0.38 Bq/m3) during the same period of time (Al-Qaradawi et al. 2015). Table 1 presents the concentration of 137Cs in different marine environment in comparison to the obtained results. Data varies according to their precipitation and location to previous nuclear events.
The obtained results of the Kuwaiti Bay seawater were comparable to the data reported from 15 stations in the Arabian Sea after taking into consideration the decay correction the sampling analysis collection time (IAEA 2005). It should be noted that 134Cs was not detected and falls below the detection limit (0.3 mBq L-1). High chemical recovery percentage (95-100%) in both analyses indicates the full extraction of the radioisotope with minimal loss. These values can be considered low compared with the recent 137Cs average concentration measured in the Western North Pacific in March 2011 (3.39 Bq L-1)(Men et al 2015). Obviously, the low data of 137Cs in Kuwait Bay can be attributed to the indirect impact of 137Cs fallout in the Gulf area; it was transported and produced abroad, in addition to the absence of the regional nuclear applications and incidents in the region.
Table 1. Average values of 137Cs on 01-01-2000 in different worldwide locations (IAEA 2005).
Conclusion
This study examined specific activity concentrations of radioactive Uranium and Cesium in Kuwaiti marine water, which were regarded as low and equal to the provincial marine environment. The obtained concentration values can be utilized as a reference for the continuous environmental monitoring program to be developed in the country. The calculated 234U/238U isotopic ratio confirms that the impact of the second Gulf war is insignificant and can be considered negligible. According to the reported results of 137Cs in dust and marine sediment, the major sources of Cesium in Kuwait Bay come from the bomb test experiments in the Northern Hemisphere, with a minor contribution from Chernobyl accident. The dust fallout plays a major role in transporting 137Cs in the form of dust from Turkey as well as Syrian and Iraqi deserts. Ultimately, the obtained data reflects the radiological safety of Kuwait marine, namely, the locally sourced desalinated water and seafood consumption. These experimentally determined levels of 137Cs, 234U, and 238U pose no ecological risks, and they seem to be informative for further radiation-monitoring program of the local marine environment.
Reference List
Aba A, Al-Dousari A, Ismaeel A (2018) Atmospheric deposition fluxes of 137Cs associated with dust fallout in the northeastern Arabian Gulf. J Environ Radioact 192:565-572.
Aba, A et al (2014) Radiometric dating of sediment records in Kuwait’s marine area. J Radioanal Nucl 301.1: 247-255.
Al-Shemmari H, Al Senafy M, Al-Fayad K (2002) Effects of seasonal variations on the water quality in Kuwait bay. Proceedings of the International Conference on Coastal Zone Management and Development, EPA, Kuwait V1-V4.
Al-Ghadban A, Saeed T, Al-Dousari AM, Al-Shemmari Al-Mutairi, HM (1999) Preliminary assessment of the impact of draining of Iraqi marshes on Kuwait’s northern marine environment. parti. physical manipulation. Water Sci Technol 40.7: 75-87.
Al-Zamel A, Bou-Rabee F, Olszewski M, Bem H (2005) Natural radionuclides and 137Cs activity concentration in the bottom sediment cores from Kuwait Bay. J Radioanal Nucl Chem 266.2: 269-276.
Al-Qaradawi I, Abdel-Moati M et al (2015) Radioactivity levels in the marine environment along the Exclusive Economic Zone (EEZ) of Qatar. Marine Pollution Bulletin 90.1-2:323-329.
Bajoga AD, Alazemi N, Regan PH, Bradley DA (2015) Radioactive investigation of NORM samples from Southern Kuwait soil using high resolution gamma-ray spectroscopy. Radiat Phys Chem 116: 305-311. Web.
Biegalski SR, Hosticka B, Mason LR (2001) Cesium-137 concentrations, trends, and sources observed in Kuwait City, Kuwait. J Radioanal Nucl Chem 248.3 643-649.
Carter, MW, Burns, P (1993) Radiotoxicity hazard classification-the basis and development of a new list. 2nd version. Australian government publishing service, Australia.
Chabaux F, Riotte, J, Clauer N, France-Lanord C (2001) Isotopic tracing of the dissolved U fluxes of Himalayan rivers: implications for present and past U budgets of the Ganges– Brahmaputra system. Geochim Cosmochim Acta 65.19:3201-3217.
Dovelte, C, Povince PP (2004) Quantification of uncertainty in gamma-spectrometric analysis of environmental samples. Int At Energy Agency 35.40:103-126.
Huang D, Deng F, He J, Yu T (2016) Determination of uranium in seawater, biological samples and sediments using laser induced fluorescence spectrometry. J Radioanal Nucl Chem 307:1359-1363.
IAEA (2005) Worldwide Marine Radioactivity Studies (WOMARS): Radionuclide levels in oceans and Seas, IAEA-TECDOC-1429.
IAEA (2009) A Procedure for the Rapid Determination of Po-210 in Water Samples by Alpha Spectrometry. IAEA (2010) Analytical Quality in Nuclear Applications Series No. 12. IAEA/AQ/12.
Kamenik J, Dulaiova H, Sebesta F, Stastna, K (2013) Fast concentration of dissolved forms of cesium radioisotopes from large seawater samples. J Radioanal Nucl Chem 296:841-846.
Khalaf F, Al-Hashash M (1983) Aeolian sedimentation in the northwestern part of the Arabian Gulf. J Arid Environ 6.4: 319-332.
Lebanese Atomic Energy Commission – National Council for Scientific Research (CNRS) (2010) Lebanon Upgrading Regional Capability to Assess Marine Contaminants in the ARASIA Member States. IAEA Publishing.
Mamish S, Al-Masri MS, Durgham H (2015) Radioactivity in three species of eastern Mediterranean jellyfish. J Environ Radioact 149:1-7.
Men W, Jianhua H, Wang F (2015) Radioactive status of seawater in the northwest Pacific more than one year after the Fukushima nuclear accident. Scientific Report 5.7757: 1-9.
Miyake Y, Sugimura Y, Uchida T (1966) Ratio U234/U238 and the uranium concentration in seawater in the western North Pacific. J Geophys Res 71.12: 123-129.
Monty C (2001) UNSCEAR Report 2000: sources and effects of ionizing radiation. J Radiol Prot 21.1:83-85.
NIH (2005) Compound summary Cesium-137. Pubchem publishing.
Yusof NF, Sharib J, Jaffary NA Md (2016) Determination of 238PU and 239+240PU in marine sediment using alpha spectrometry. Eurasian J Anal Chem 12.4:405-416. Web.
UNSCEAR (2000) Sources and effects of ionizing radiation: sources-volume i: sources. United Nations Scientific Committee on the Effects of Atomic Radiation. United Nations. Web.
Uddin S, Al Ghadban AN, Aba A, Behbehani M (2012) Concentration of selected radionuclides in seawater from Kuwait. Marine Pollution Bulletin 64.6:1261-1264.
Uddin, S, Fowler SW, Aba AM, Behbehani, M (2015) Radioactivity in the Kuwait marine environment—baseline measurements and review. Marine Pollution Bulletin 100.2:651-661.
WHO (2001) Depleted Uranium sources, exposure and health effects. World Health Organization. WHO/SDE/PHE/01.1, Geneva.