Introduction
The use of instrumental analysis methods is a routine practice for laboratory work, which helps reduce systematic error and obtain more accurate results. The present experiment used spectroscopic methods of analysis based on the trajectory of electromagnetic radiation rays and the absorption and reflection processes of such rays, which has practical utility for investigating the characteristics of the substance under study.
In particular, in the center of focus was ultraviolet radiation spectrometry, the essence of which is reduced to the irradiation of the sample with ultraviolet waves of a given character to study the absorption parameters. This work aims to identify the concentration of an unknown substance based on a calibration curve, which was obtained by spectroscopic study of substances of known concentration. Conducting this laboratory work allows for practicing spectroscopic research methods and gaining valuable experience working with a spectrometer under laboratory conditions.
Method
In the first part of the present work, the objective was to determine the wavelength of electromagnetic radiation in the UV-visible light spectrum at which the maximum absorption by a substance is observed. Three pre-prepared dye solutions were presented for which lambda measurements were performed. First, distilled water was added to a 3-mL cuvette and used as a background to calibrate the spectrophotometer. In the next step, lambda measurements were performed for each previously transferred solution into the respective 3-mL cuvettes.
In the second part of the laboratory work, the task was to construct a calibration curve from the data of a known solution and then calculate the concentration of an unknown solution. To do this, after calibrating the spectrophotometer on distilled water, three KoolAid solutions with a given concentration and colors were measured at 430 nm, and the data were used to construct the calibration curve. The absorbance of the KoolAid solution with unknown concentration was then measured. Beer’s law determined the concentration based on the empirically measured absorbance.
Data and Results
The first part of the laboratory work involved studying the maximum absorbance lengths for the three dye solutions; the data obtained are summarized in Table 1. These results were used to set up a spectrophotometer to measure KoolAid solutions with a given concentration.
The table represents the results of direct measurements of the maximum absorption length on a spectrophotometer for the three dyes and their colors.
Table 2 represents the results of the empirical determination of absorbance for three KoolAid solutions whose concentrations were known in advance — it is this data that was used to construct the calibration curve (Figure 1). As is known from the Beer equation, there is a linear relationship between absorbance and solution molarity, which has been proven for empirical data (Harvey, 2023). The coefficient of determination of the regression model was 0.99, indicating that the linear approximation is critically accurate. The coefficients of the equation show that at zero molarity, the absorbance is 0.0665 (which is the error of the regression model) and that when the molarity is increased by one unit, the absorbance increases by 0.6258 absorbance units.
Measurements were made for KoolAid with a known concentration in 2X increments. As can be seen from the data, the concentration of the substance in solution determined the color saturation.
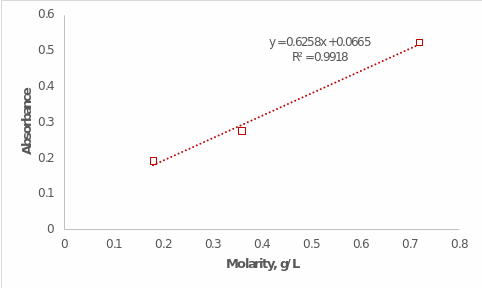
The calibration curve was used to estimate the concentration of the KoolAid solution with unknown molarity. Specifically, absorbance measurements showed a value of 0.394 units: substituting this value into the regression equation instead of Y gives the following molarity value:
Thus, the molarity of the KoolAid solution of unknown concentration using the calibration curve was shown to be 0.5233 g/L.
Discussion
The present laboratory work was to investigate the practicality of measuring the concentration of a solution using spectrophotometric methods. The results showed the success of empirical determination using a calibration curve — specifically, the concentration of KoolAid was determined to be 0.5233 g/L. Although the laboratory work was performed successfully and the objective was achieved, limitations cannot be ruled out. First, reading errors could have occurred when writing the results, which could have affected the results. Second, linear approximation does not rule out the presence of errors that also distort the accuracy of the final result.
Conclusion
The present laboratory work was based on using spectrophotometric methods to estimate the KoolAid concentration of a solution of unknown concentration. The calibration curve construction method was used and showed excellent feasibility for application. In addition, the coefficient of determination of the constructed model showed high agreement with Beer’s law, which also proves the success of the present laboratory work.
Reference
Harvey, D. (2023). 13.2: Beer’s law. LibreTexts. Web.