Introduction
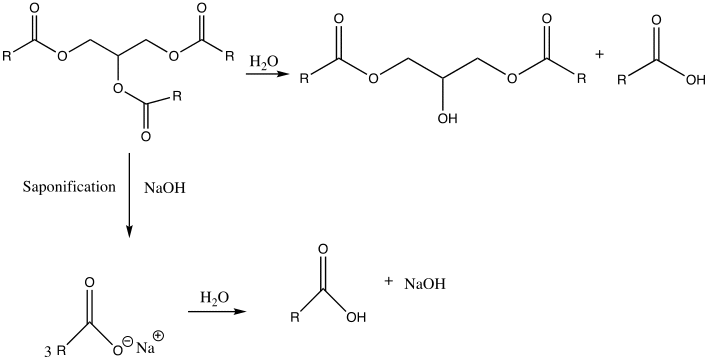
Biodiesel and soap are made through transesterification and saponification. Saponification is the process of reacting a triglyceride with an aqueous hydroxide ion to form glycerol and fatty acid salts (Weldegirma). It is used to manufacture soap.
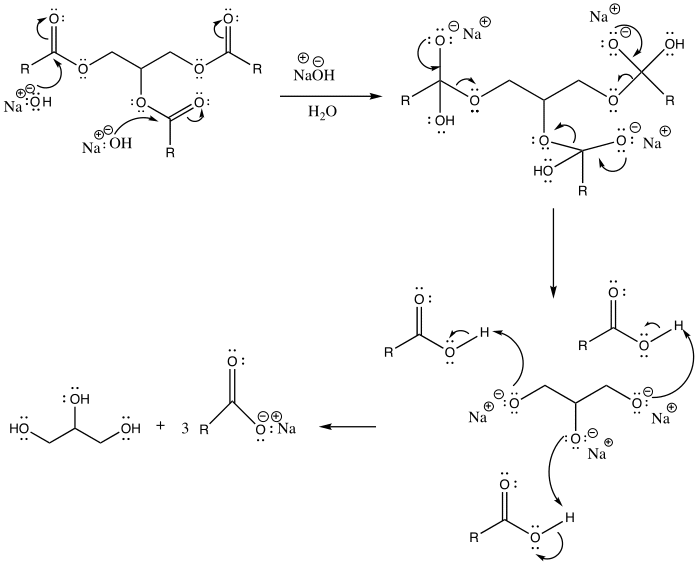
Biodiesel is a renewable alternative to diesel fuel produced from biological sources, including animal fats and vegetable oil. It is made via a transesterification reaction. This is a chemical process by which an organic group in an ester is substituted with another organic group obtained from alcohol. The reaction occurs in the presence of an acidic or basic catalyst. (Weldegirma). The addition of a catalyst should be carefully done to ensure that the ester is not completely hydrolyzed, hence making the soap more corrosive.
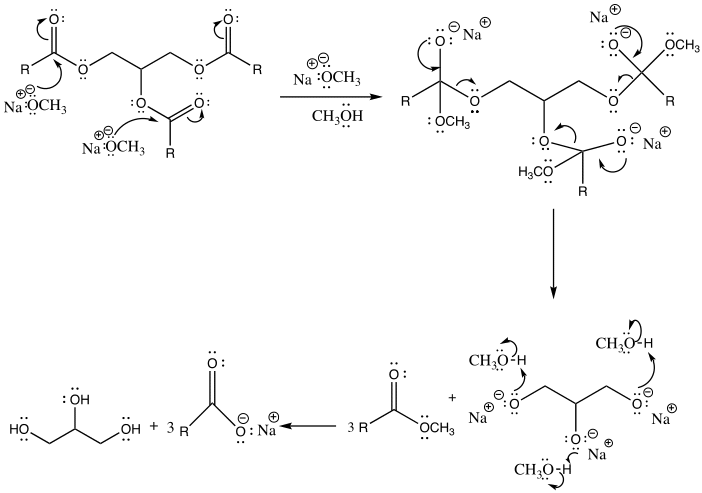
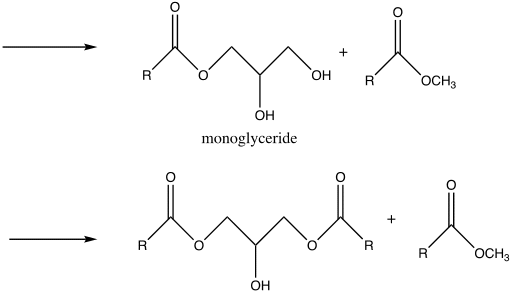
Nevertheless, there are potential side reactions that ensue during transesterification and saponification. In the case of transesterification, the side reactions are dependent on a carbonyl attack by a methoxy group, conversely, for saponification, the carbonyl is attacked by a hydroxide ion in the case of saponification. As a result, due to electron transfer, the double bond between the oxygen and carbon atoms is converted into a single bond (Weldegirma). Consequently, the ester bond breaks in the three fatty acid chains and glycerol are formed.
Experimental Section
Synthesis of biodiesel
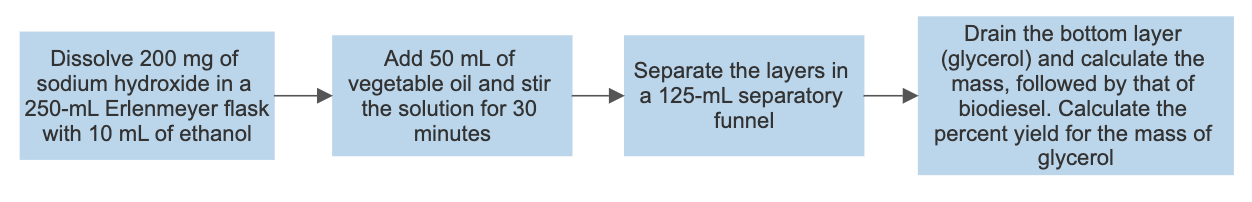
Synthesis of soap
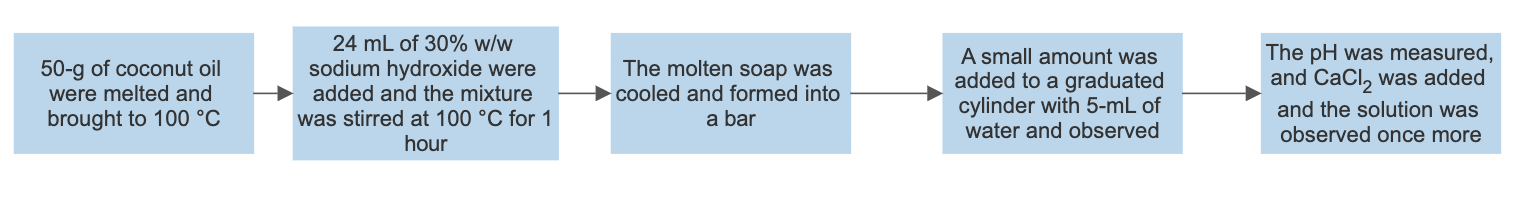
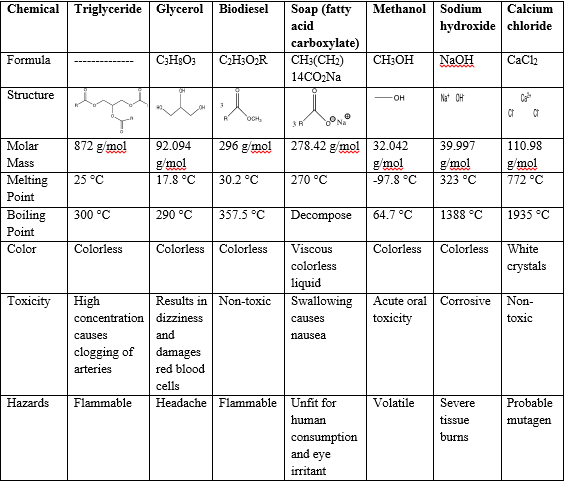
Table of Chemicals
Results

Discussion
Based on the observations, the reaction between the triglyceride in coconut oil and sodium hydroxide resulted in the formation of soap (fatty acid carboxylate). For instance, upon cooling the aqueous solution, a white solid with a pleasant smell was formed. Furthermore, the end-product tested positive for soap when it produced foam upon dissolving in water. Its purity is also confirmed when the pH strip turned green. This indicates that the product was amphiphilic, which is, it possessed both lipophilic and hydrophilic properties (Weldegirma). However, if the pH strip had turned blue, this would mean that a lot of sodium hydroxide had been added; therefore, the resulting soap solution would have been caustic. Lastly, depending on the nature of the alkali used in their production, soaps have distinctive properties. A white solid was formed upon the addition of calcium chloride, indicating that “hard soap” was formed. The soap solution reacted with calcium chloride to form calcium stearate (scum).
Biodiesel was produced from the triglyceride as the end-product comprised of a clear yellow and viscous liquid. Hence, this illustrates that vegetable oil is a suitable source of renewable energy. This occurred by the process of transesterification, where the triglyceride reacted with methanol in the presence of a sodium hydroxide catalyst to form a methyl ester (biodiesel) and glycerin. Glycerin is denser than biodiesel, as explained by the two layers formed after the reaction. The amount of oil obtained was relatively high, 42.367g, suggesting that vegetable oil is a good biodiesel source. However, it tested a negative for the 27/3 methanol test, suggesting that the product was not pure biodiesel. This deviation might be due to several errors during the experiment and potential side reactions. For instance, the reactants were not stirred sufficiently, the temperature was too high or too low, or the sample was contaminated. It is essential to point out that both saponification and transesterification entail reacting a triglyceride with alcohol using a hydroxide as a catalyst. However, the difference lies in the presence or absence of methanol as a reactant that results in different nucleophilic attacks, hence specific products (Weldegirma).
Conclusion
This experiment shows that vegetable oil is a good source of biodiesel as it produces a high quantity of the product, while coconut oil is a good source for soap as it produces non-caustic soap. These are economical and environmentally unstable raw materials; hence, they are regarded as suitable alternative fuels from biomass sources. These oils contain triglycerides, which, when subjected to different experimental processes (saponification and transesterification), form different products. Nevertheless, depending on the concentration of the reactants, such chemical processes are vulnerable to potential side reactants that interfere with the final product’s purity. For instance, the biodiesel formed was impure; thus, it is recommended to stir the solution longer than one hour at 60 °C.
Work Cited
Weldegirma, Solomon. Experimental Organic Chemistry. 8th ed., University of South Florida, 2018.