Introduction
There are a number of reasons defining the importance of correct evaluation of various mutagenic reactions in chemical compounds. Although many similar tests were designed, at the moment, the Ames test remains to be the most efficient for distinguishing mutagenic activities. Unlike several other options of assessing a mutagenic potential of certain chemical compounds, the Ames test does not require much time to run and is not costly or harmful for the environment. Overall, the Ames test functions on the basis of observing and controlling the development of bacterial cells, as they convert from the state of histidine auxotrophy to the state of histidine prototrophs.
It is important to point out that there are several ways to find out whether a certain chemical compound is able to cause such mutation. The classic application of the Ames test includes using the organisms of Salmonella typhimurium. It is a strain of Salmonella obtained from rat liver, and it needs to be able to synthesize histidine in order for mutation to be possible. During the process of interaction with a possible mutagen, if a mutation happens, histidine will be required for the mutated bacterial cell to grow. After that, the process includes a number of reverse mutations, in which case Salmonella strains under analysis will enter the state of revertant. In other words, because of the fact that there is not enough histidine, they will become prototrophic cells again. Therefore, the number of revertants discovered during the Ames test will indicate mutations.
Hypothesis
In the process of the Ames test analysis, several chemical compounds were tested on a subject of their potential mutagenicity. All of the tested chemicals were nitro polycyclic aromatic hydrocarbons. It means that there is a high possibility of their mutagenicity. Two compounds that were tested against Salmonella strains were nitrofurazone (NFZ) and 2-nitrofluorene (2-NF). Two different Salmonella strains were YG -1026 and YG-1021. Those Salmonella strains had genes HisG and HisD respectively.
The primary hypothesis is that both nitro-furazone (NFZ) and 2-nitrofluorene (2-NF) are mutagenic when tested on Salmonella strains and that mutations could not be considered as merely spontaneous ones.
Because of a high number of mutations occurring in Salmonella strains YG -1026 and YG-1021, it is reasonable to suggest that the mutations are not accidental, but they indicate mutagenicity of the given chemical compounds.
Results
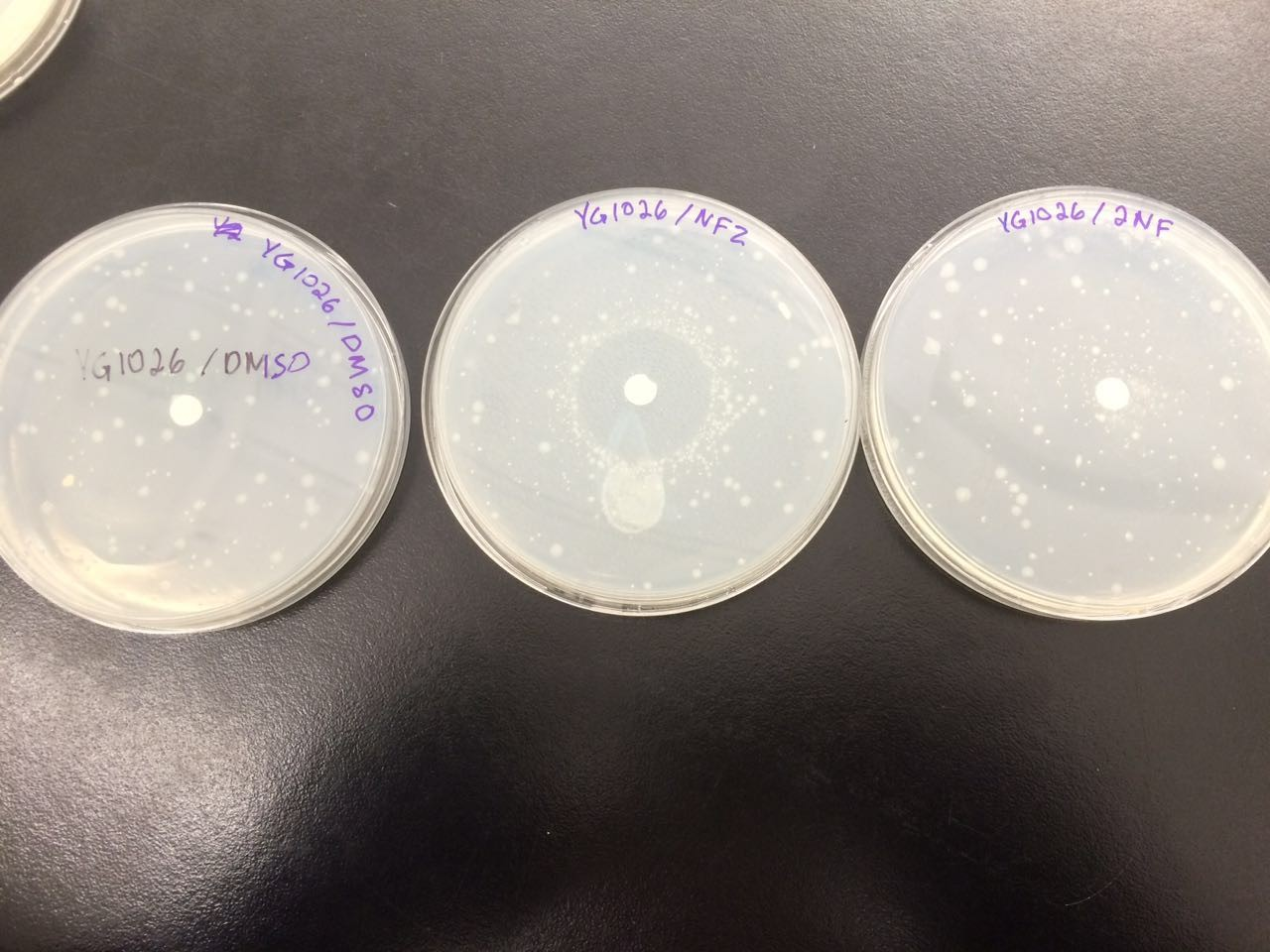
The following figures 1 – 4 represent the interaction of several Salmonella strains YG -1026 and YG-1021 with nitrofurazone (NFZ) and 2-nitrofluorene (2-NF). On the basis of the results, it would be possible to count the revertant colonies and define whether mutations are spontaneous or they are the result of mutagenic chemical compounds.
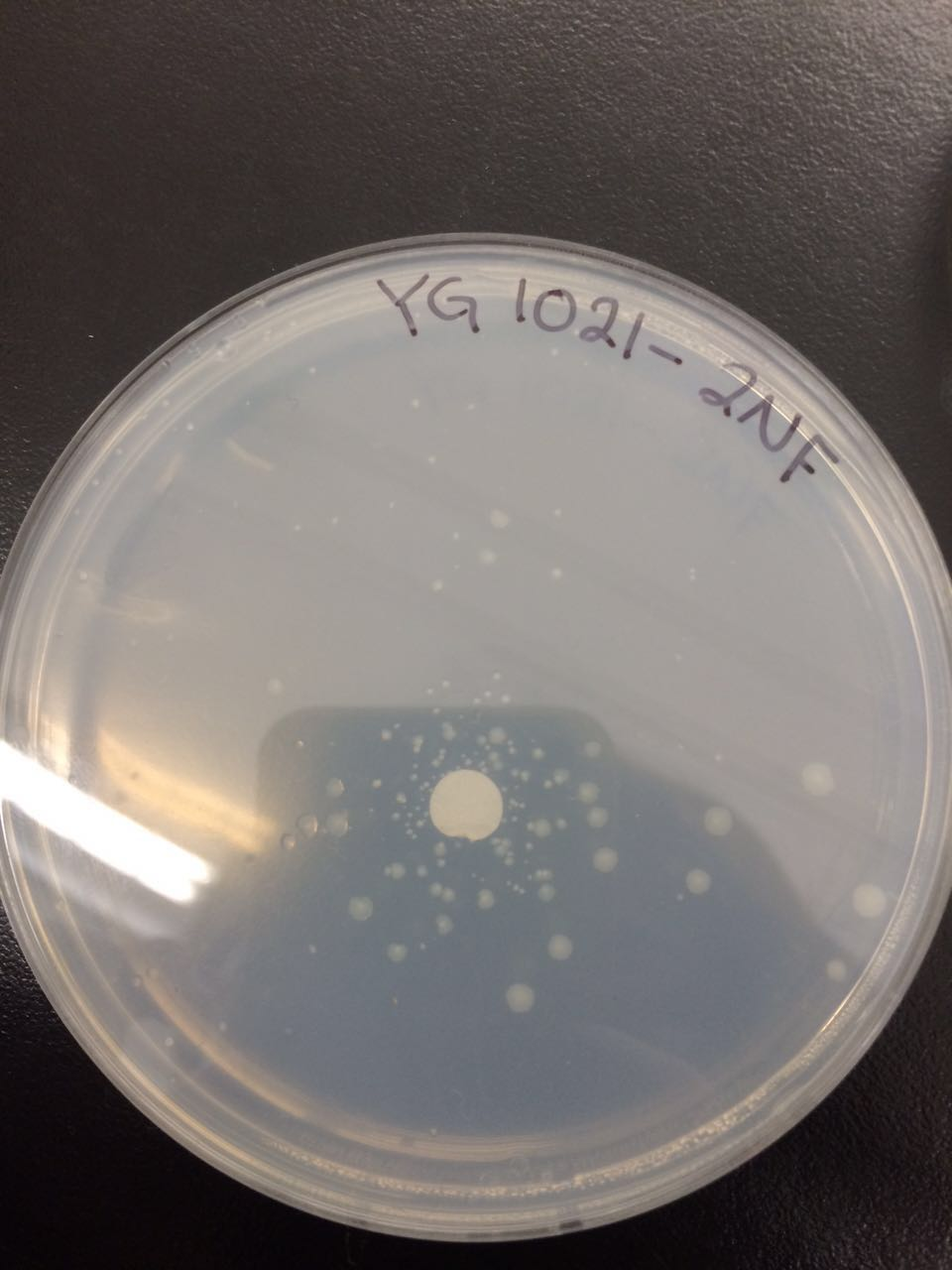
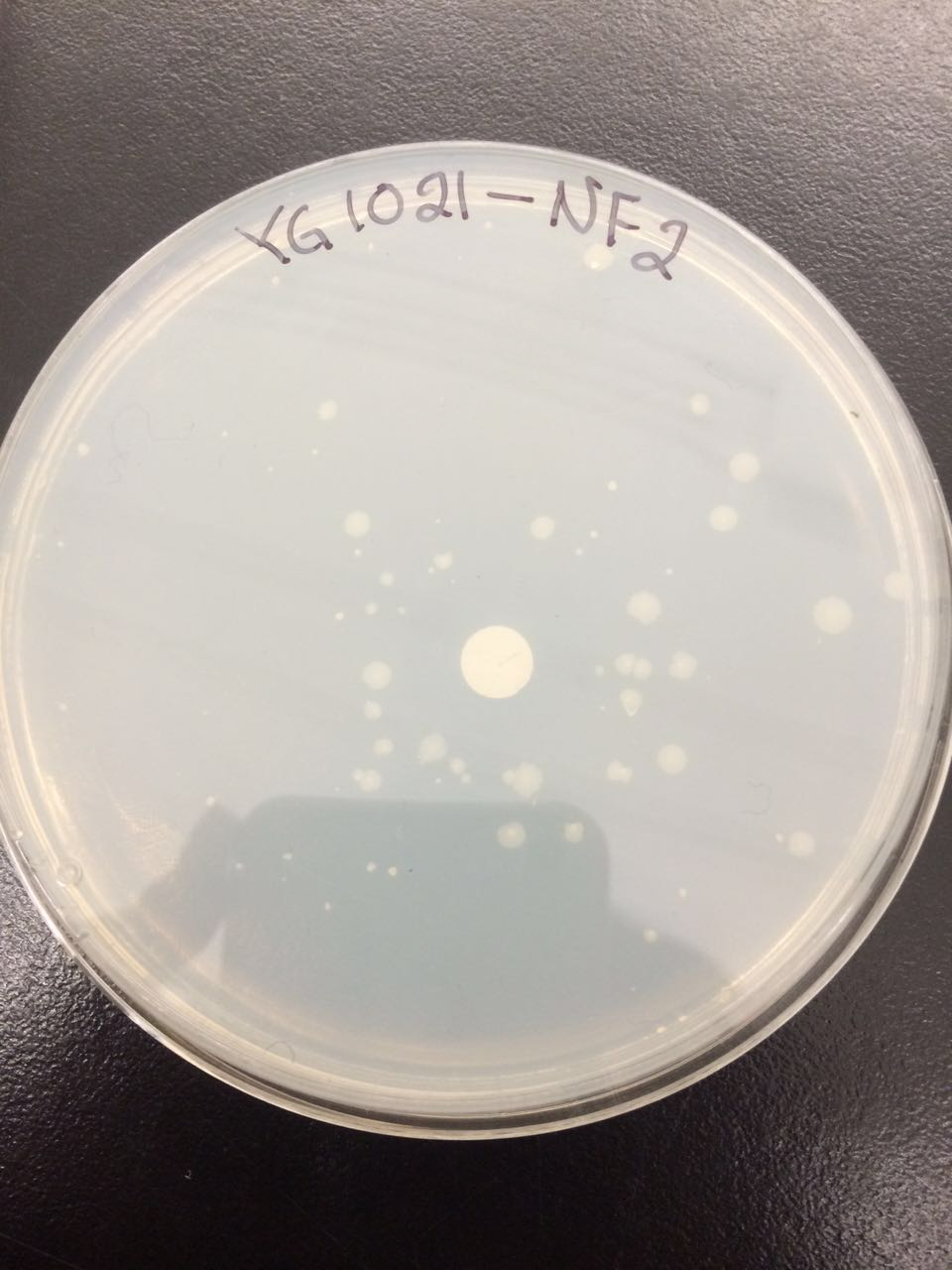
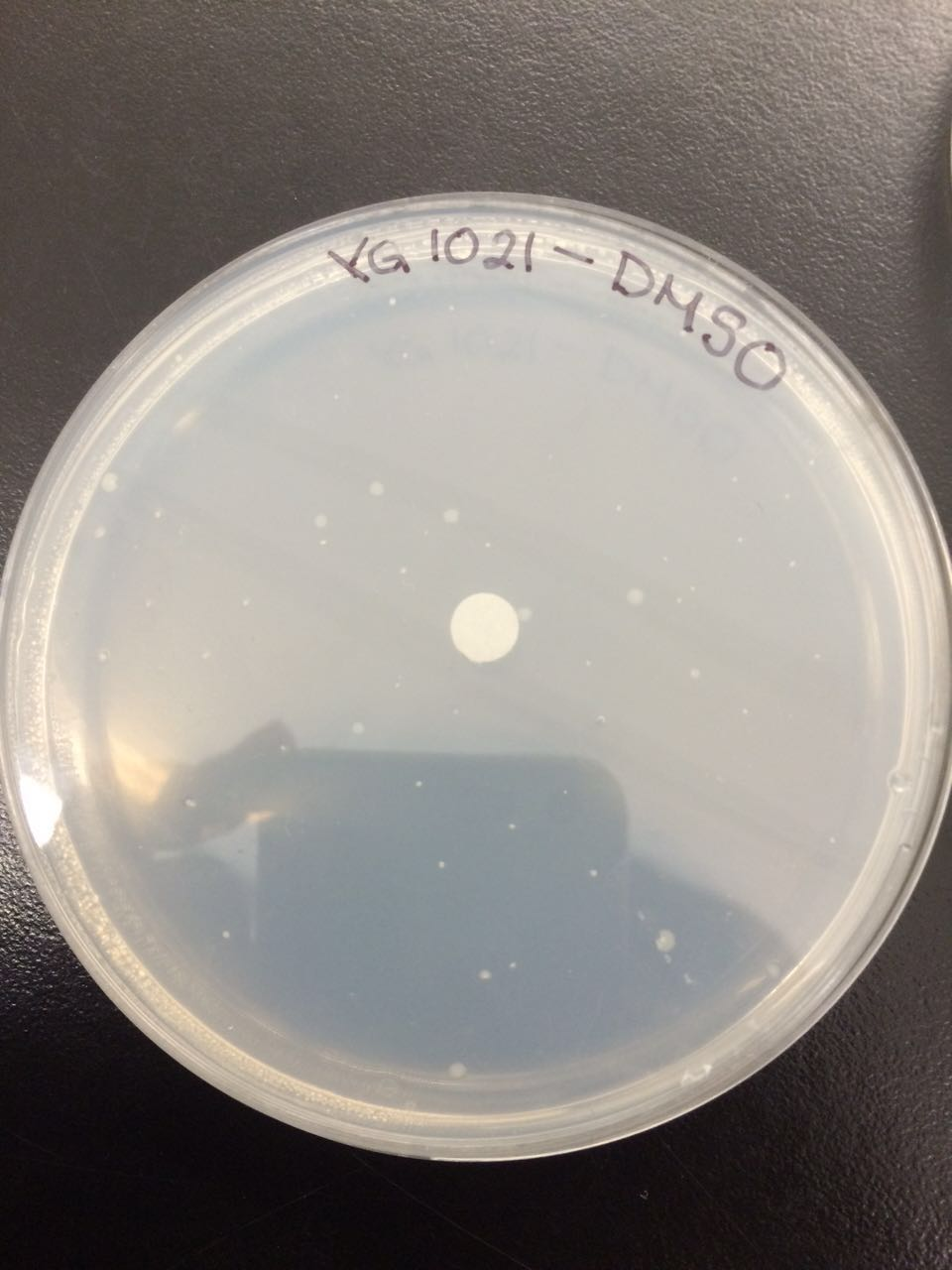
According to the figures 1 – 3, the YG-1021 strain reacting with 2NF got 45 colonies, whereas the same strain on a plate of NFZ generated approximately 61 colonies. The spontaneously mutated strain YG-1021 has only produced approximately 22 colonies.
Figure 4 shows that the YG-1026 strain with NFZ generated more than 200 colonies. The 2NFplate with the YG-1026 strain produces around 110 colonies. The spontaneously mutated YG-1026 strain had 70 colonies.
Discussion and conclusions
Overall, the YG-1026 has more revertant colonies than the strain YG-1021both after reactions with chemical compounds and as a result of spontaneous mutation. It means that it is more likely to mutate under any circumstances. The hypothesis was proved, and the Amis test was effective in defining mutations.