Objective and Overview
The goal of this lab is to produce a sample of pure aspirin crystals. Towards achieving this, the experiment utilizes the reaction of the salicylic acid with acetic anhydride, which yields aspirin and acetic acid. The word and chemical equation for the reaction can be illustrated as follows:
Salicylicacid + Acetic anhydride → Aspirin + Aceticacia
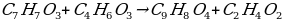
Concentrated sulfuric acid is utilized to speed up the reaction.
Apparatus and Reagents
- Salicylic acid
- Acetic anhydride
- Concentrated sulfuric acid
- 600ml beaker
- 100ml beaker
- Ethanol
- 125ml conical flask
- 100ml conical flask
- 10ml measuring cylinder
- Teat pipette
- Ice cubes
- Rough balance
- Buchner apparatus
- Aspirator
- Ring stand
- Iron clamp
- Filter paper
- Glass stirring rod
- Rubber policeman
- Deionized water
- Thermometer
- Stopwatch
Preparation of the Crude Aspirin
- Create a thermostatic water bath by adding 100ml of water into a 600ml beaker and heating it to about 700C, as shown below.
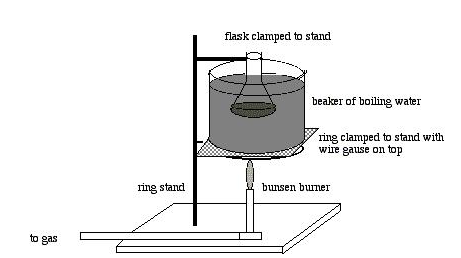
- Weigh approximately 4.0g of salicylic acid in a 125ml conical flask and record it.
- Using the 10ml measuring cylinder, add 5ml of acetic anhydride to the conical flask.
- Using the teat pipette, add approximately 5 drops of the concentrated sulfuric acid to the conical flask.
- Swirl the mixture slightly and place the conical flask in the hot water bath. Ensure the conical flask is clamped to a ring stand, constant stirring with a glass rod for the complete dissolving of the solids, and the mixture heats up for about 10 minutes.
- Take out the conical flask from the water bath and keep it uninterrupted to permit crystallization at room temperature.
- When crystal formation stops, add and thoroughly twirl about 20ml of ice water to precipitate out the insoluble aspirin and tear down the acetic anhydride that failed to react.
- Transfer the solution to a 100ml beaker in readiness for product dissociation from the mixture.
Aspirin Separation from the Mixture
- Use the Buchner apparatus to sieve off and collect the aspirin crystals through the vacuum filtration process.
- Set up the Buchner apparatus as below by clamping the side-arm conical flask to the ring stand. Fix one end of a rubber tube to the side-arm and the other end to the aspirator. Insert a Buchner funnel into the flask, fasten it with a rubber stopper and fix an appropriately fitting filter paper. Make sure the paper is rinsed with distilled to ensure it sticks to the apparatus.
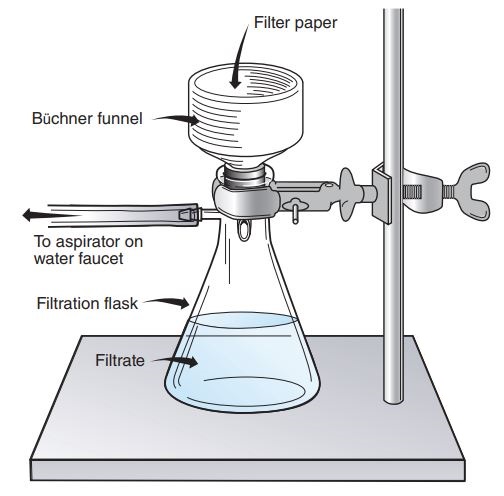
- Switch on the aspirator to full blast, whirl the flask with aspirin and pour it on the filter paper. Using the rubber policeman, pickle out the superfluous precipitate in the flask. Rinse the sample on the filter paper with chilled water and leave set up to run for about 5 minutes.
- Wash the obtained aspirin with 5ml of cold water for at least three times, followed by a single 5ml of ethanol to do away with the impurities.
- Let the crude aspirin desiccate, use the rough balance to weigh it, and record the mass.
Purification of Aspirin via Recrystallization
- Measure about 5ml of ethanol using a measuring cylinder, add it to 100ml conical flask and warm it to 700C.
- Using a spatula, add about 3g of the collected crude aspirin into the flask and warm the mixture until all crystals dissolve.
- Add about 10ml of deionized water to ensure all particles dissolve and allow the solution to cool slowly in an ice bath.
- Upon completion of crystallization, amass the pure aspirin crystals via the vacuum filtration and allow them to dry.
Risk Assessment for the Experiment
- The practical should be carried out in a fume hood as the material used are hazardous.
- Protective clothing like goggles and gloves should be worn as acetic anhydride causes an irritating effect on the eyes and skin.
- No flame should be used to heat the ethanol as it is highly combustible.
- All the waste products and materials should be deposited in the organic waste bins.
- The aspirin synthesized in this practical is impure and should not be injected.
Works Cited
“Chemistry 104: Synthesis of Aspirin”. Chem.Latech.Edu, 2020, Web.
Csun.Edu, 2020, Web.
Laney.Edu, 2020, Web.
Montinari, Maria Rosa, Sergio Minelli, and Raffaele De Caterina. “The first 3500 years of Aspirin History from its Roots–A Concise Summary.” Vascular pharmacology 113 2019: 1-8.
Roberts, Tracey et al. “Acetylsalicylic Acid (Aspirin) for Schizophrenia”. Cochrane Database of Systematic Reviews, 2016. Wiley, Web.