Introduction
Thermochemical reactions are an important object of study in general chemistry, allowing the study of thermal processes that occur during chemical interactions. It is well known that the whole set of reactions can be divided into exothermic and endothermic, depending on the thermal effect: heat can either be released or absorbed (LibreTexts, 2021). In this regard, of particular interest is the study of enthalpy or the energy that a system possesses at constant pressure. In other words, enthalpy characterizes the heat content of a thermodynamic system. As a consequence, in the case of exothermic reactions, enthalpy is negative, whereas a positive change in value is characteristic of endothermic reactions.
This principle was the central idea for the present laboratory work, in which heat effects were measured for three neutralization reactions. It was shown that all reactions were exothermic, which means that during the interactions, some heat was released. The purpose of this laboratory report is to describe the measures performed and to summarize the results of the experiment.
Procedure
An interactive virtual model hosted on the Beyond Labz portal was used to perform this laboratory work. The initial temperature of the acid was determined using a built-in thermometer. To a beaker containing 100 mL of 1M hydrochloric acid solution in the calorimeter were added 100 mL of 1M sodium hydroxide solution: the temperature change was not made immediately but after twenty seconds of temperature stabilization. A similar procedure was performed for the interaction of weak acid with sodium hydroxide and sulfuric acid with sodium hydroxide. All volumes and concentrations of the reagents were unchanged. Table 1 was used to record the results.
Results
Initial and final temperatures after neutralization stabilized twenty seconds after mixing were measured for the three experimental lines. Summary Table 1 numerically describes these thermodynamic transformations.
Table 1. Initial data on the temperature change in a series of three neutralization reactions.
The above results were sufficient to calculate the heat effect of each of the three reactions. For this purpose, the critical thermodynamic formula given in Equation 1 was used. It should be noted that since the water was used as the solvent in the reactions, the specific heat constant of the solution and the density to calculate the mass were carried out through water characteristics. Once the value of the total heat of the reaction had been calculated for all three processes, it was necessary to determine the enthalpy of the reactions. Equation 2 was used for this purpose: the number of moles was calculated through the concentration of the acid or alkali solution: since the concentrations were the same, there was no difference. A summary of all the final results is given in Table 2, while an example of the calculations for the second experimental line, the interaction of weak acid and sodium hydroxide, is given in Equations 3-4.
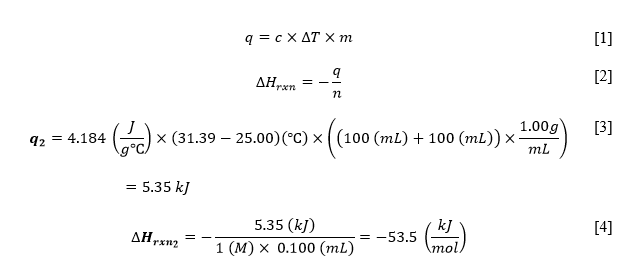
Table 2. Indirect calculations of total heat and enthalpy change for the three interactions.
Discussion and Conclusion
Thermodynamic processes have unique properties that characterize changes in heat effects during interactions. Any chemical reaction, regardless of the type, occurs with changes in the heat: this energy can either be absorbed or released during the processes. It should be specified that chemical interactions between molecules usually involve the breaking of some chemical bonds and the formation of others.
These transformations do not necessarily deal with the same bonding energy, so chemical processes describe either an increase in thermal energy or a decrease in it. In the case of the release of excess energy — when the final energy of the final bonds is lower than the energy of the initial ones — such interactions are called exothermic (LibreTexts, 2021). On the contrary, if the system requires an additional source of absorbed energy to carry out the reaction, this process is commonly referred to as endothermic.
In this laboratory work, three neutralization reactions were studied: two of them between a strong acid and a strong base and one between a weak base and a strong acid. Neutralization reactions are those processes in which the chemical potential of acid is reduced by adding a base (LibreTexts, 2020).
As a result, such interactions produce salt and water. The three thermochemical neutralization reactions studied in this work were exothermic, as evidenced by the negative values of enthalpy change. In addition, although the activity of the acids used was different, the final values of both total heat and enthalpy were similar. Most likely, the reason for this similarity is the neglect of the masses of acid and base and the use of aqueous characteristics instead.
In conclusion, it should be noted that thermochemical reactions are an essential part of general chemistry. An in-depth study of this area of knowledge allows not only qualitative determination of the type of reaction but also quantitative calculations of measurable parameters. Knowing which type of reaction a particular process belongs to — whether exothermic or endothermic — the lab technician can predict the change in heat during the interaction. In this report, it has been shown that for the three neutralization reactions, it is sufficient to know the total mass of the system, the specific heat constant, and the temperature change to determine both the total heat released and the enthalpy change. It was shown that, for the three interactions studied, these parameters are generally similar.
References
LibreTexts. (2020). Neutralization [PDF document]. Web.
LibreTexts. (2021). Exothermic and endothermic reactions [PDF document]. Web.