Background
Numerous painful conditions of patients are caused by physical damage to the integrity of the body and the possibility of dangerous pathogens entering the internal environment. Overcoming the primary protective barrier of the skin, the pathogen activates its mechanisms of attacking human cells and suppresses the functioning of the body.
This type of parasitic relationship is usually aimed at a unilateral benefit for the pathogen from the resources of the patient’s body, with the health and well-being indicators of the infected person being significantly reduced. If such a condition is left untreated, there is a high probability of complete health suppression and, as a consequence, death. To avoid the criticality of similar conditions, modern medicine offers preventive measures, among which vaccination is of great importance.
The pathogen of high research interest in this paper is a Gram-negative bacterium with the scientific name Yersinia pestis. In other words, it is the bacillus that causes the dangerous disease state of the plague, which several centuries earlier exterminated more than twenty million Europeans. By deeply studying the nature and mechanism of attack of this pathogen, medicine has learned to create a drug that inhibits the development of Yersinia pestis in the infected body, so if patients have the appropriate vaccination, they do not have to worry about the disease.
Epidemiology of Yersinia pestis
Plague, which is a manifestation of the pathogenic activity of the bacillus in the host organism, is an acute infectious disease of the quarantine group, expressed as severe intoxication, deep lesions of the skin, lymph nodes, lungs, and other organs. Furthermore, the clear visualization of the patient’s infectious infection is the presence of buboes, or critically inflamed lymph nodes, as shown in Figure 1. After passing the incubation period, which usually lasts no more than a few days, the Yersinia pestis pathogen activates two antiphagocytic antigens, F1 and VW, allowing the bacteria to destroy the body’s blood cells (Sangwan, 2016). Although the unique mechanism and order in which organs become infected depend on how the infection enters the body, the general pattern of disease development is characterized as a sequential suppression of vital organs.

The spectrum of symptomatic manifestations of plague bacillus is wide and depends on the severity of the infectious disease course. As a rule, infected persons may complain of pain in the muscles, joints, and sacral area. Subsequently, bloody vomiting and an excruciating feeling of thirst appear (“Plague,” 2019). From the first hours, patients are agitated, and perceptual disturbances, including delusions or hallucinations, may be noted. Patients become uncoordinated and lose basic cognitive functions: speech, perception, or critical thinking. In some cases, lethargy and apathy may occur, with the patient becoming weak to the point of inability to get out of bed.
The infection mechanism in healthy humans is due to transmission of the pathogen from a rodent: thus, the plague is an anthropozoonotic infection. In particular, wild rodents, especially gophers, are natural reservoirs of the bacterium, but certain wild hares, cats, or predators may also be sources of infection. In the context of epidemiological prevalence, the transmissible transmission of the bacterium is worsened by outbreaks or even pandemics. The human body comes into contact with Yersinia pestis via fleas and by contact, food, and respiratory routes. In addition, secondary contaminated objects and patient cadavers pose a high risk.
Morphology of the Bacterium
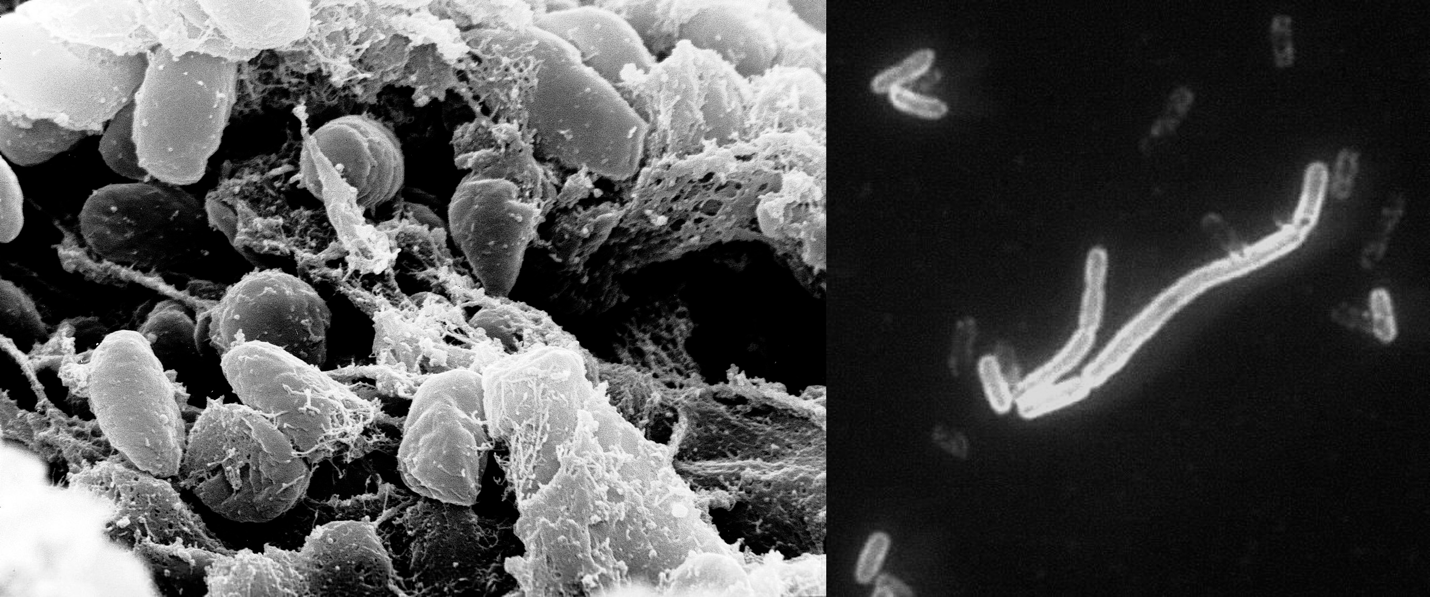
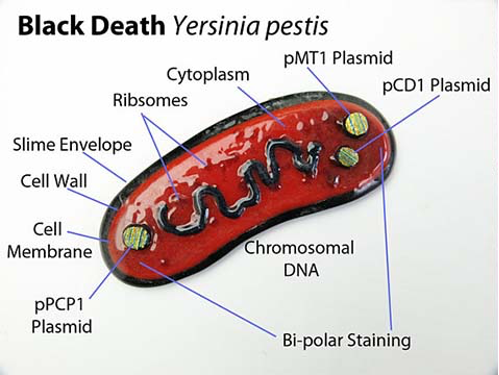
In terms of taxonomy, the plague pathogen belongs to the bacterial family Enterobacteriaceae, genus Yersinia, and biological species Yersinia pestis. It is an immobile facultative anaerobic Gram-negative bacilliform prokaryote with linear dimensions not exceeding 0.5-0.8 µm by 1-3 µm, as illustrated in Figure 2 (Bhunia, 2020). Morphologically, the bacterium has a very delicate protein capsule that protects the microorganism from external stimuli and phagocytosis (Figure 3). Such features of the membrane, however, dictate the possibility of polymorphism of the plague bacillus: the bacteria form filamentous, bulbous, or globular forms. The bacteria have neither spores nor flagella, which justifies their attached lifestyle.
Biochemical Characteristics of the Pathogen
The metabolism of the plague bacillus is oriented toward saccharolytic activity. Yersinia pestis is characterized by the enzymatic cleavage of numerous carbohydrates with the formation of corresponding acids. In particular, many biochemical tests show that the pathogen tends to break down many sugars, including glucose, dextrin, galactose, mannose, arabinose, or maltose (Aryal, 2018; Expert ASM, 2016).
Two pathogen biotypes are distinguished concerning glycerol, namely glycerol-positive and negative ones. Nevertheless, it is worth noting that plague bacillus is incapable of fermenting rhamnose, sucrose, gelatin, does not produce indole compounds and hydrogen sulfide, cannot reduce nitrates, and does not break down urea (Saini, 2020). Because of the cytoplasm’s irregular distribution, the ends of the bacilli stain more intensely than the middle part. This bipolarity is clearly visible when stained with methylene blue.
The health hazards of the plague bacillus effect are due to the production of a biological toxin. It is a protein molecule that combines the properties of exo and endotoxin, consisting of two polypeptide fractions that differ in amino acid composition and antigenic properties (Monnappa et al., 2018). Entering the host through the skin, mucous membranes of the respiratory tract, or digestive tract, Yersinia pestis penetrates the bloodstream and activates immune cell inhibition processes, provoking sepsis.
In terms of resistance to environmental factors, the plague bacillus can remain viable for a long time in human excreta and cadavers. In bubonic pus, Yersinia pestis can survive for up to 20-30 days, and in the cadavers of humans and dead animals for up to 60 days, it also survives freezing. In addition, there are reports that the bacilli can change into cysts under unfavorable conditions and remain inactivated for twenty years (Markman, 2018). This bacterium is quite sensitive to environmental factors such as sunlight, atmospheric oxygen, heat, changes in the medium’s acidity, or disinfection. As an anaerobe, plague bacillus shows the greatest cultural activity under optimal temperature conditions of 27-28 degrees and a pH of 6.9-7.1.
Replication Characteristics
The replication mechanisms of the bacterium differ little from classical prokaryotic patterns. Yersinia pestis effectively infects the host’s neutrophils and macrophages through cellular phagocytosis (Demeure et al., 2019). The surviving bacterium localizes in autophagosomes, from where it provokes replication of its own genetic material. This process is accompanied by an active release of the cytokine IL-1RA, which stimulates a decrease in the body’s immune response combined with the pathogen’s increased biochemical activity.
In the natural reservoirs of the bacteria, in fleas, the esophagus forms a thickening, a goiter, in front of the stomach. The plague bacteria entering the flea body when bitten by an infected animal multiply intensively there and secrete the enzyme coagulase (Sebbane et al., 2020). As a result, a plug is formed in the goiter, preventing blood from entering the stomach. The plague-blocked flea is constantly hungry and moves from one host to another in the hope of getting a portion of blood. Thus, a constant circulation of the pathogen along the “rodent→flea→rodent” chain ensures the natural focality of the plague.
Genome
It should be additionally noted that Yersinia pestis differs from other representatives of Yersinia by the presence of two characteristic plasmids. These include pMT1 and pPCP1, which are capable of encoding polypeptides that provoke flea transport of the bacterium and ensure effective infection of host cells (Demeure et al., 2019). Such features are encoded at the genomic level of the prokaryote (“Yersinia pestis,” n.d.). More specifically, the Yersinia pestis genome is known to contain 4,068 genes actively coding for 3,843 proteins with an average size of 4.55 Mb. At the same time, the GC content does not exceed 47.6%.
Treatment and Prevention
Considering this pathogen’s high contagiousness, constant control of natural foci, and organization of measures to prevent human diseases are critically necessary. For precise prevention of plague, a specially developed vaccine is used, which develops specific immunity in the patient’s body. In case of disease, regardless of the form, strict isolation in the hospital’s infectious disease department is necessary. A diet and control of fluid intake are recommended. Upon arrival at the clinic, the patient is prescribed antibiotic therapy from tetracyclines, fluoroquinolones, or aminoglycosides.
References
Aryal, S. (2018). Biochemical test of Yersinia pestis. Microbe Notes.
Bhunia, A. K. (2020). Yersinia enterocolitica and Yersinia pestis .
Clamp, R. (2020). Coronavirus and the Black Death: Spread of misinformation and xenophobia shows we haven’t learned from our past. The Conversation.
Demeure, C. E., Dussurget, O., Fiol, G. M., Le Guern, A. S., Savin, C., & Pizarro-Cerdá, J. (2019). Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes & Immunity, 20(5), 357-370.
Expert ASM. (2016). Sentinel level clinical laboratory guidelines for suspected agents of bioterrorism and emerging infectious diseases.
Markman, D. (2018). Plague bacteria may be hiding in common soil or water microbes, waiting to emerge. The Conversation.
Monnappa, A. K., Bari, W., Seo, J. K., & Mitchell, R. J. (2018). The cytotoxic necrotizing factor of Yersinia pseudotuberculosis (CNFY) is carried on extracellular membrane vesicles to host cells. Scientific Reports, 8(1), 1-8.
Nyirenda, S. S., Hang’ombe, B. M., Mulenga, E., & Kilonzo, B. S. (2017). Serological and PCR investigation of Yersinia pestis in potential reservoir hosts from a plague outbreak focus in Zambia. BMC Research Notes, 10(1), 345-351.
Pagadala, J. (2020). Plague and its forms caused by Yersinia pestis. The Biology Notes. Web.
Plague. (2019). Mayo Clinic.
Saini, V. (2020). Yersinia , Pasteurella and Francisella . Web.
Sangwan, A. (2016). Yersinia pestis in humans | systematic bacteriology. Biology Discussion.
Sebbane, F., Uversky, V. N., & Anisimov, A. P. (2020). Yersinia pestis Plasminogen Activator. Biomolecules, 10(11), 1554-1586.
Yersinia pestis. (n.d.). NCBI. Web.