Introduction
In microbiology, there are a tremendous number of microorganisms that are important to human life. Many are naturally parasitic forms and cause harm, but symbionts are essential to study. When a microorganism lives inside the human body and consumes its resources, offering practical support in return, clinicians must know as many details of its existence as possible. Any abnormalities can cause systemic damage, with health being the first to suffer as a result. One such symbiont of particular biological and academic importance is E. coli.
It is worth saying that E. coli is a typical prokaryotic bacteria of bacilliform shape. The absence of nuclear structures and the presence of the nucleus as an alternative to a formalized nucleus confirm that E. coli belongs to the prokaryote class. Recent studies show that DNA, the primary genetic molecule of the bacteria, contains 5 Mb and about 4,200 genes within the genome (ANU, 2018). The other organelles of the bacterium are also typical: E. coli has a membrane, cytosol, and cell wall. The bacterial cell wall is composed of polysaccharides, particularly structures lined with pectin and cellulose (Ziege et al., 2021). Concerning the cell membrane, it would also be fair to say that it is represented by several layers with a thin core of peptidoglycan — based on these words, one can conclude that E. coli is a textbook example of Gram-negative bacteria not stained with crystal violet. In terms of dynamics, the bacterium moves to utilize peritrichial flagellate-like outgrowths, which reactively force the microorganism to move in the medium.
Usually, E. coli is a free-living organism, but it can form colonies, many with a metallic sheen when cultured on media. In terms of shape, E. coli are primarily straight bacilli, but some may have slightly curved shapes. The size of the average E. coli ranges from two to thirty micrometers (Wu et al., 2019). For this reason, the bacteria can be observed under a conventional optical microscope. As the Latin name makes clear, the natural habitat of such bacilli is the intestinal system of animals. Moreover, the bacterium adheres to the inner walls of the lower intestine, thus being a facultative anaerobe. Some bacterial serotypes can remain viable for several months in soil and water, but they are not inclined to form spores. However, prolonged exposure to oxygen or direct sunlight causes immediate death of the bacterium.
Virulence Factors
Although it has been said before that E. coli is symbiotic to humans and benefits them by synthesizing certain types of vitamins, any imbalance of this bacterium or specific serotypes can cause severe damage to gastrointestinal health. Pathogenicity occurs when E. coli leaves its natural intestinal environment by accidentally entering other body cavities. Virulence factors are any cellular structures and molecules that allow the bacteria to initiate its pathogenicity toward the host. In the case of E. coli, it would be correct to say that its main pathogenicity factors are adhesion, endo- and exotoxins, and substances promoting invasion. CFA adhesins, which are fimbriae that contribute to the attachment of the bacterium to human cell receptors, are found in the bacillus cell (Rollenhagen et al., 2019). Bacterial adhesiveness reinforces their physical stability in the environment and inhibits the ability to repopulate the gut with competitive parasites, which creates an advantageous ability for E. coli. Once the bacterium has been attached to the receptors, it triggers the previously expressed invasion proteins. Typically, such proteins in E. coli are represented by the membrane structures of intimin and T3SS, which can trigger polymerization reactions for host cell lysis processes; this facilitates the infiltration process. Finally, among the virulence factors of the bacterium are the toxins that the bacterium produces to kill host cells and thus cause health damage. E. coli exotoxins include any enterotoxin-type toxins that modify the permeability of the cell membrane, causing the host cell to die. Bacterial endotoxins are represented by lipopolysaccharide, which affects the coding of E. coli antigens. These include hemolysin, CNF, and colicins, which promote preferential development of the bacterium in the affected area. In particular, this causes damage to the intestinal blood vessels, which becomes the cause of inflammation.
Immunity
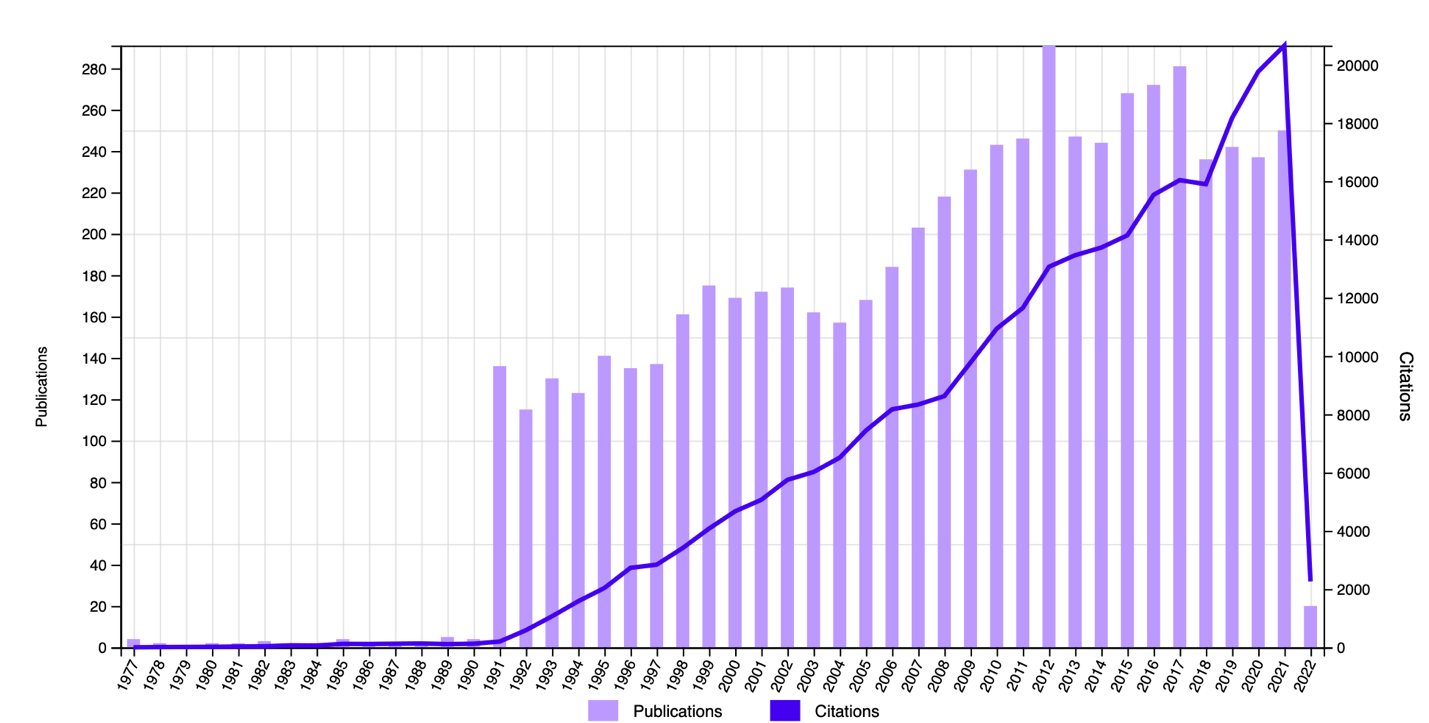
The affected body will not ignore any inflammation, so an immune response is triggered at the first signs, even if not yet symptomatic. In this sense, there is some overlap since most of the immunity is concentrated in the individual’s gut; this is where E. coli develops. However, despite the overlap, the immune response mechanism to E. coli has not been well characterized, as long-standing studies show (Li et al., 2000). In this sense, it is reasonable to say that colonization by E. coli occurs during the first days of the infant’s life, and since no states of gastrointestinal dysbiosis are observed at this time, it can be assumed that the type of immune response to the bacterium is innate. This judgment is confirmed by the symbiotic relationship between the bacterium and man — as a result of long years of evolution, the H. sapiens organism could adapt to living together with the pathogen. Thus, from the immune point of view, research on the pathogen is actively pursued (Fig. 1) until its mechanism of action becomes clearer.
Pathology
When E. coli is imbalanced or present in atypical areas, a pathological condition occurs in the body. The WHO estimates that E. coli causes a 3-5% mortality rate of infection, with the presence of other diseases, especially of a chronic nature, significantly increasing the risks of a severe disease course (WHO, 2018). E. coli is capable of causing dysbiosis, a condition in which a purulent inflammatory process begins in the patient’s intestinal system. Inflammation is localized to where the infection begins, so there is no universal tissue affected by the pathogen. If the lesion occurs in the intestinal tract, E. coli parasitizes on the epithelial cells of the inner intestinal wall, causing the development of purulent inflammation there. Such a disease is called Escherichiosis after the term of the bacteria itself, and the individual experiences abdominal cramps and diarrhea, general weakness in the body, vomiting, and fever. As a rule, it takes up to ten days from the moment of infection to the appearance of the first symptoms, which is the incubation period of pathogenesis. It is noteworthy that E. coli is the latent form because it is already in the body but does not cause an immune response. However, it is worth noting that some E. coli serotypes are even capable of causing an autoimmune reaction, provoking an unwanted immune response to a symbiotic bacterium. Thus, E. coli provokes predominantly intestinal lesions but, when dislocated, is capable of causing urogenital tract inflammation and even meningitis (Zhao et al., 2018). Consequently, neglecting treatment can lead to severe health damage, with establishing the cause taking time to perform a serologic smear.
Epidemiology
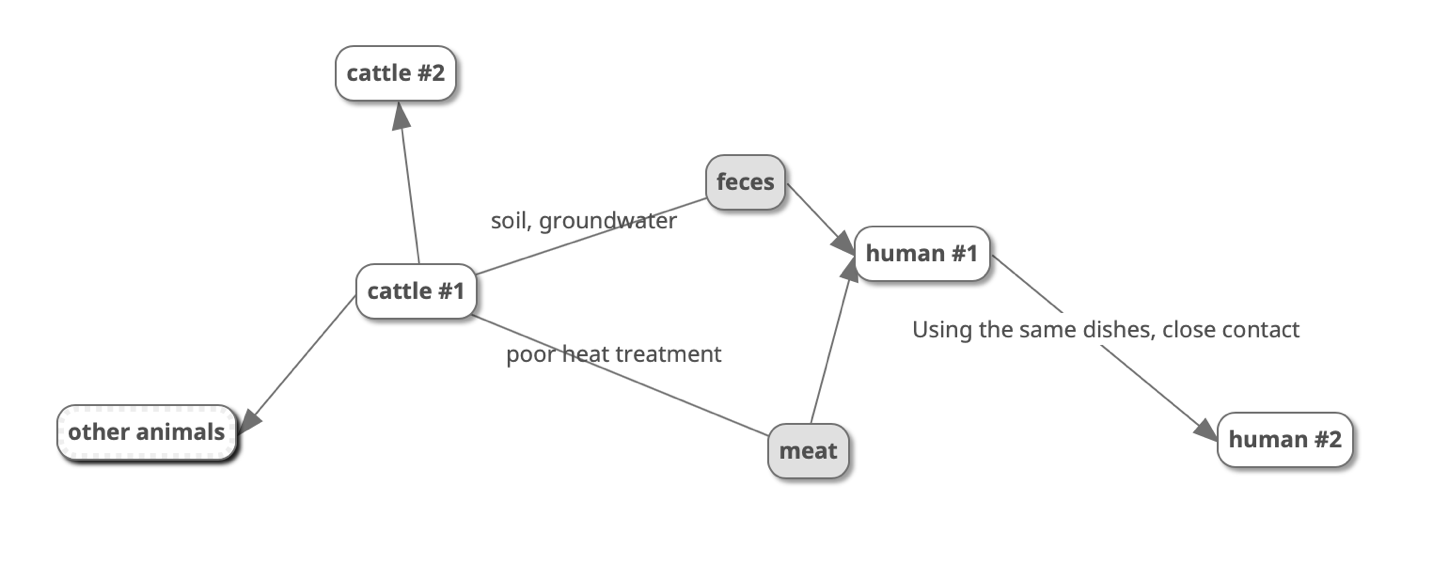
Infection of the human body with E. coli occurs primarily through cattle (a natural reservoir), along with their feces and undercooked meat and meat products. Unwashed hands after handling soil and farm water can cause infection, as shown in Figure 2. Notably, cattle can infect humans and all trophically related animals, whether birds or rodents, with bacteria. A person can also infect another person if they have close contact through the oral-facial transmission mechanism. The patient can release some of the parasitic pathogens by the fecal route, but the strong adhesion of the bacteria to the intestinal walls does not facilitate this; in addition, even the fact of finding the serotype in the feces does not mean it is eliminated from the body. Thus, hot climates, poor farm sanitation, personal hygiene, active travel, and contamination of foodstuffs contribute to the development of the epidemic. According to official data, the epidemiology of E. coli is based on predominantly infecting patients from high-income countries (WHO, 2018). This could probably be due to either the development of a semi-raw meat diet and contamination of urban sewage water or the prevalence of beef as a meal in general. An intestinal infection has also been determined to be mediated by the age of the patients: the probability of escherichiosis increases significantly with aging, from 0.00048 at age 55 to 0.003 for those over 75. E. coli is also thought to cause up to 50% of all hospital-acquired and out-of-hospital patient urinary tract infections (Madappa, 2019). Thus, this E. coli causes a prevalent pathological condition, which means that the clinical system has already developed effective mechanisms for prevention and treatment.
Prevention
Because of the symbiotic association of the organism with E. coli, universal and effective vaccines that would apply to children are still not available. Vaccination efficacy studies are ongoing, but it is already clear that the vaccine is not the only preventive option (Garcia, n.d.). The best preventive practice against E. coli infections is to follow personal hygiene and sanitation practices at the production level. First, hands should be thoroughly washed with aseptic soap before contact with mucous membranes (eyes, nose, ears). Second, any potentially contaminated food is recommended to be heat-treated and washed with detergents designed for food. Finally, the strictest sanitary controls are required at the production level, with constant checks by a sanitation doctor. If pathogenic bacteria are found, production is suspended completely, and products are recalled.
Treatment
Treatment aims to eliminate the threat with antibiotic agents and restore the body’s water-salt balance. For E. coli, Levofloxacin, Ciprofloxacin, and sulfamethoxazole are recommended to inhibit the growth and reproduction of the bacterium (Madappa, 2019). However, it is worth saying that modern medicine is more inclined not to prescribe any drug treatment for mild escherichiosis because it can complicate the course of the disease (MCS, 2020). Instead, it is recommended to consume plenty of clear fluids and avoid physical activity.
Clinical Significance
With the widespread use of antibiotics, serotypes with resistance to many drugs have emerged among E. coli strains. Studies have found that E. coli can horizontally divide genes using plasmids to promote bacterial resistance in a colony (Sanger, 2021). This news allows certain strains (ST131) of the pathogen to be assigned superbacterial status, a significant challenge for clinicians and public health (University of Birmingham, 2019). ST131 has become widespread in the population and hospital patients in recent decades, and no reliable drug treatments for the bacterium have been developed (Nicolas-Chanoine et al., 2014). One of the basic practices is to withhold medication in cases of mild disease while initiating the continued search for new antibiotic formulations.
References
ANU. (2018). Evolution of genome size in E. coli. Australian National University. Web.
Bonten, M., Johnson, J. R., van den Biggelaar, A. H., Georgalis, L., Geurtsen, J., de Palacios, P. I.,… & Poolman, J. T. (2021). Epidemiology of Escherichia coli bacteremia: a systematic literature review. Clinical Infectious Diseases, 72(7), 1211-1219. Web.
Garcia, A. (n.d.). The E. coli vaccine and its effects [PDF document]. Web.
Li, Y., Frey, E., Mackenzie, A. M., & Finlay, B. B. (2000). Human response to Escherichia coli O157: H7 infection: antibodies to secreted virulence factors. Infection and Immunity, 68(9), 5090-5095. Web.
Madappa, T. (2019). Escherichia coli (E coli) infections. Web.
Madappa, T. (2019). Which medications in the drug class antibiotics are used in the treatment of Escherichia coli (E coli) infections? Web.
MCS. (2020). E. coli. Mayo Clinic. Web.
Nicolas-Chanoine, M. H., Bertrand, X., & Madec, J. Y. (2014). Escherichia coli ST131, an intriguing clonal group. Clinical Microbiology Reviews, 27(3), 543-574. Web.
Sanger. (2021). Identifying the rise of multi-drug resistant E. coli. Sanger. Web.
Rollenhagen, J. E., Woods, C. M., O’Dowd, A., Poole, S. T., Tian, J. H., Guebre-Xabier, M., … & Savarino, S. J. (2019). Evaluation of transcutaneous immunization as a delivery route for an enterotoxigenic E. coli adhesin-based vaccine with CfaE, the colonization factor antigen 1 (CFA/I) tip adhesin. Vaccine, 37(42), 6134-6138.
University of Birmingham. (2019). How ‘superbug’ E. coli clones take over human gut. Science Daily. Web.
WHO. (2018). E. coli. WHO. Web.
Wu, F., Swain, P., Kuijpers, L., Zheng, X., Felter, K., Guurink, M.,… & Dekker, C. (2019). Cell boundary confinement sets the size and position of the E. coli chromosome. Current Biology, 29(13), 2131-2144.
Zhao, W. D., Liu, D. X., Wei, J. Y., Miao, Z. W., Zhang, K., Su, Z. K.,… & Chen, Y. H. (2018). Caspr1 is a host receptor for meningitis-causing Escherichia coli. Nature Communications, 9(1), 1-16. Web.
Ziege, R., Tsirigoni, A. M., Large, B., Serra, D. O., Blank, K. G., Hengge, R.,… & Bidan, C. M. (2021). Adaptation of Escherichia coli biofilm growth, morphology, and mechanical properties to substrate water content. ACS biomaterials science & engineering, 7(11), 5315-5325.