Introduction
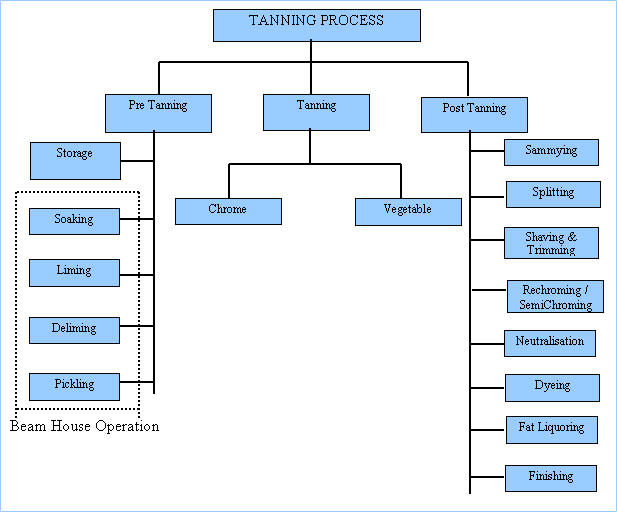
Industrial activities are essentials for the quality of life because the products derived from the same makes life worth living. The dawn of the 21st century has illuminated myriads of industrial activities. The majority of the countries with a considerable GDP are highly industrialized. As a trend towards development, the industrial establishment is seen as the ultimate vehicle for the same. Thus industrialization is a target for every nation. It is being encouraged in every nation. One major industrial activity is tanning. Tanning is the process of converting skins and hides into useful components. The products from the same are used for several purposes and as a raw in shoe industries. The tanning process is an extensive industrial process. It involves mixing, cutting, trimming, and washing, among many other processes. The figure below indicates a glimpse of what happens inside the tanning factory.

Tanning Industry and Environmental Pollution
The tanning industry, as seen from above, is indispensable to human life. However, the industry impacts negatively on the environment. The tanning process yields much waste that is disposed of as wastewater into the rivers. In most cases, tannery waste results in environmental pollution. The wastes from the same normally have a pungent smell, blocks water drainage pipes, increase the BOD and COD, and may harm the aquatic life. The majority of the industries do not mind environmental pollution; they discharge the tannery waste into the nearby river without treatment and thus causing pollution. An example of pollution from the tannery industries is shown below.

Solution to the Problems of Tannery Waste Pollution
The wastes from tannery industries are inevitable. The world cannot do without tanneries; neither can the world also content with environmental pollution, yet the tanneries also cannot function without realizing wastes. This has called for a focus on converting the waste from the tannery into a form that can be contained in the environment. The concern of the environmentalist has been to come up with the best treatment method for tannery waste treatment that has no further side effects to the surroundings. This is because the existing treatment methods have not been to the required standards. For instance, the chemical treatment methods though effective, have been found to be harmful to the environment. This means that the focus should be on the best method for treating tannery waste. This paper investigates an alternative method for tannery waste treatment. The method involves the treatment of tannery wastewater using Chitosan, a naturally existing polymer that has minimal side effects (Srivastava & Thakur 2006, p.1169).
Chitosan
Chitosan is a complex form of glucose that is of the polysaccharide formula. It is made of randomly distributed NH2 units. The chemical structure is shown below.
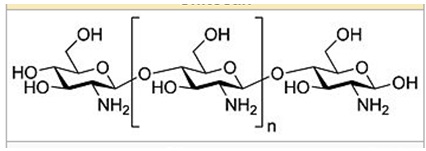
Chitosan is derived from the exoskeletons parts of the crustacean. Crustaceans are sea animals such as crabs and shrimp. In addition, the cell wall of fungi is also used to produce Chitosan. “The structural element of the exoskeleton and cell walls of fungi contains chitin, which is taken through a chemical process known as deacetylation in the commercial production of Chitosan” (Ates, Orhon & Tunay 1997). The synthesis process takes time through a chemical bath that is allowed to react on that path (Kean, Roth & Thanou, 2005, p. 643). Chitosan structure has an amine group (NH2) that has a PKa value of about 6.5. This means that protonation occurs in acidic to a neutral solution with the charge density depending on the PH value. This property is what makes Chitosan soluble in water (Mazumder, Mukherjee & Ray 2008, p.45).
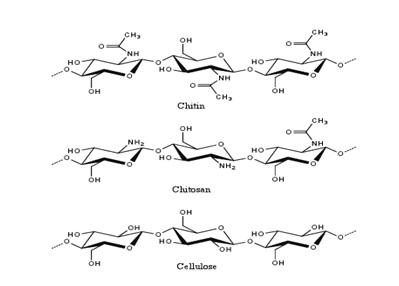
In the organic nomenclature of hydrocarbons, a double polymer or biopolymer is grouped as chitin or Chitosan, depending on the degree of deacetylation. Deacetylation is determined by the degree of D-glucosamine and N-acetyl-Glucosamine. Chitosan has a structure that is similar to the one of cellulose and is the most popular and basic bipolar. It has only one monomer, the one of glucose. As seen in the first paragraph, Chitosan is soluble. On top of that characteristics, it is also biodegradable, reactive, and absorbent. The degree of the mentioned characteristics depends on the quantity of protonated amino groups in the polymeric chain and hence the proportion of the Acetylated and non-acetylated D-glucosamine units. Chemically, the amino acids with a pKa value ranging from 6.2 to 7.0 are entirely protonated in acids with a pKa value of below 6.2. This property makes Chitosan soluble in acids. Chitosan is not soluble in water, bases, and organic solvents (Shakoori, Makhdoom & Haq 2000, p.349). However, it becomes soluble in the mentioned solvents after string it in acids such as acetic acid, nitric acids, hydrochloric acids, phosphoric acids, and perchloric acids. The solubility comes as a result of protonation. The figure below indicates the structures of Chitosan, chitin, and cellulose.
Chitosan harbors some properties that make it the most valuable polymer in the application of biomedical and pharmaceutical. The polymer is antimicrobial, meaning that it fights against microorganisms such as bacteria. This property can be harnessed in food industries for preservation. Another important property is the non-toxicity. This property implies that Chitosan cannot contaminate the medium in which it is placed, which makes it safe for human beings and also animals. This means that the polymer can be used in water and foods without fear of toxicity or contamination (Sumathi, Mahimairaja & Naidu 2005, p.315). This property makes it possible for Chitosan to be used in water purification and food preservation. Another property of this polymer is the property of biodegradability. This implies that the polymer is environmentally friendly. When disposed to the environment, it will decompose. Other property includes anti-tumor and biocompatibility (Wang, Pan, Lang, Xu & Zheng 2007, p.321).
Tannery Effluents and Their Effects on the Environment
Leather is an important part of the manufacture of different items. It forms the main material for the production of shoes and bags and many leather items. Leather is obtained from animals, fish, or birds. It is obtained in its crude form, meaning that some processing must be done on it to render it effective for use in factories (Szpyrkowicz, Stern & Grandi 1991, p.1354). The processing of leather, also called tannery, involves several steps with by-products released as effluents, as shown in the diagram below.
The tanning process normally yields a lot of wastes. The complete process involving wastes is as shown below.
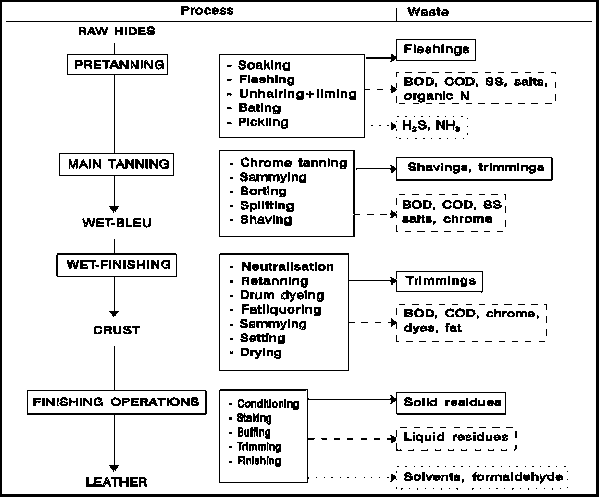
Although leather is good for many processes, the effluents are a nuisance to the environment. The following are the effluents obtained from the tanning process.
Solid tannery wastes
Solid wastes from tannery effluents can be grouped into several categories as follows.
Suspended solids
These are components of insoluble matter found in tannery effluent (wastewater). They are problematic to the environment when discharged. They essentially consist of two characteristics. The first one is settleable solids.
Settleable solids
These are solids with rapid settling rates. They comprise materials seen in suspension when the sample of the effluent is shaken but quickly settle when the sample is left still. Settling time is about five to ten minutes, although in some cases, the settling may take even an hour and above. Settleable solids originate from all stages in the leather making process (Haydar, Aziz & Ahmad 2007, p.63). These solids wastes normally manifest as sludge in the waste treatment plant and are normally problematic in the treating process. If this sludge is discharged into water bodies, it will form a blanket in the water bodies and then deprive the aquatic plants and animals of oxygen, leading to death (Banu & Kaliappan 2007, p.21)
Semi Colloidal Solids
These are fine solids that cannot settle down even if left for a considerable time. However, they can be filtered from effluent waste. These solids are obtained from the proteins from the beam house operations. These solids do not directly cause Sludging problems. However, when broken down by bacteria over an extended time, they settle down as settleable solids and thus causing Sludging problem when discharged into the river (Banas, Plaza, Styka & Trela 1999, p.453).
Gross Solids
Gross solids are large and visible. They do not require sampling materials to isolate them. They are visible, and thus their effects are common and dangerous to the environment. The components in the waste that normally give rise to environmental problems are leather pieces of leather cuttings, trimmings, and shavings (Bosnic, Buljan & Daniels, 2000, P. 2).
Nitrogen Waste
Nitrogen in tannery effluents is contained mainly in two forms, as shown below.
Total Kjelgahl Nitrogen (TKN)
The waste from the tannery plant is mostly protein in nature. When protein is broken down, it gives rise to amino acid; Amino acid is highly nitrogenous in nature. This implies that wastes from the tannery effluent are nitrogenous in nature, and when released into the water, they pose a great problem. There are two problems posed by nitrogenous waste. The first one is the overstimulation of the growth of plants. Nitrogen stimulates growth under normal conditions (Milstein, 2003, p.56). However, the nitrogen in tannery effluent is in execs of the normal amount, and thus it overstimulates the growth of algae and other plants. Such a growth is not healthy because the moment those plants die, they pose a problem such as clogging and thus an environmental hazard (Srivastava et al. 2008, p.138). Another way in which nitrogenous wastes pose a problem to the environment is through the creation of oxygen demand. Nitrogenous waste in the tannery waste is broken down into ammonia to give out nitrogen gas and water. The products given out are not harmful to the environment, but the process itself consumes a lot of oxygen, and thus creating oxygen demand (Bosnic, Buljan & Daniels, 2000, P. 9).
Ammonium Content as Nitrogen
The tanning process moves in stages. One such stage is called liming. Liming yields ammonia, which is toxic to the environment.
Sulphide (s2)
The tannery effluent also contains a sulfide component. The sulfide results from the dissociation of sodium sulfide and sodium hydroxide compounds that are used during the tanning process. The sulfide component also comes from the breakdown of hairs during the process of removing hairs (Khan, Kawaja, Khan, Ghani & Kazmi 1999, p.41). Sulfide components pose a great danger to the environment when released in the effluent. In one such problem, the sulfide components remain in principal insoluble in the alkaline conditions. However, when the PH of the solution drops below 9.5 values, the situation changes as sulfide evolves and floats into the surface of the water. This condition causes a bad smell to the ambiance like that of a rotten egg (Bosnic, Buljan & Daniels, 2000, P. 10).
In another instance, the toxicity of the gas poses a great danger to human beings. Exposure to even a small level causes nausea and headache and, in large quantities, may cause death. In yet another instance, sulfide forms hydrogen sulfide, which is moderately soluble in water. This means that when realized to the atmosphere, it is dissolved by rainwater forming sulfurous acid, which is highly corrosive. The acid can corrode roofs, walls, and properties hence a nuisance to the environment (Bosnic, Buljan & Daniels, 2000, P. 13).
Neutral salts
There are two types of salt common in tannery effluent, as discussed below.
Sulphates (SO42-)
The majority of the chemicals used in the tanning process contain sulfate components, and thus, it is normally released in the effluent. In an attempt to remove these sulfates, aeration is done (Munz et al., 2009, p.737). However, the aeration process leads to the creation of calcium sulfate, an insoluble compound, which gives rise to some problems. One of the problems is that the sulfates cannot be completely removed by chemical means. The only possible process of removing the sulfates is through a biological process where it is bound into microorganisms. However, biological process is normally not employed, and thus the sulfate remains as it is or broken by a microorganism into stinking hydrogen sulfide (Nandy, Kaul, Shastry, Manivel & Deshpande 1999, p.121). The sulfide formed poses a danger to the metallic and concrete environment within which it is placed. If the sulfate salts are not broken down, they pose an environmental hazard because they remain as salts in the water runoff (Bosnic, Buljan & Daniels, 2000, P. 12).
Chlorides (Cl–)
During the pickling and preservation process of the hides and skins, sodium chloride is used in large quantities. The presence of sodium chloride in the tannery effluents thus forms the basis for the entry of chloride salts (Orhon, Ates & Sozen 2000). The presence of chlorides in the environment is a serious threat to the plants and bacteria. This is because chlorides inhibit plants growth. High levels of chlorides lead to the breakdown of the cell wall stricter of plants and animals. Furthermore, if the water containing chloride salts are used for irrigation purpose they inhibit plant growth by causing high evaporation rates as a result of causing surface salinity (Bosnic, Buljan & Daniels, 2000, P. 14).
Oils and Grease
The skins and hides have natural oil and grease. During tanning process, the same is released as effluent waste. Also some fatty components are produced when waste waters mingle. Oils on the surface of water blocks oxygen from entering the water and thus can kill the aquatic life. Also, fats on the surface of coagulate to form a ‘mat’ that causes blockage to the environment (Bosnic, Buljan & Daniels, 2000, P. 16).
Chromium Compounds
Tanning process also yields metallic waste. The waste from metallic components ought to be regarded with care because the wastes from the same are not biodegradable and thus pose the most threat to the environment. The most dangerous form of chromium from the tannery waste is discussed below (Bosnic, Buljan & Daniels, 2000, P. 15).
Chrome 3+ (trivalent chrome, chrome III)
The chrome tanning process yields waste containing chromium components. This happens during re-tanning and dying process where the chromium is displaced from leather (Mandal, Maity, Dasgupta & Datta 2010, p.89). Although chrome is discharged in soluble form, it undergoes a quick precipitation process when mixed with proteinous waste to form protein-chrome that adds to the sludge. The chromium is further broken down into chromium hydroxide that is stable and persists in the environment as a nuisance to the plants. Even if the chromium remains in soluble form, it is still a nuisance to the environment as it kills some microorganism such as daphnia thus disrupting the food chain (Bosnic, Buljan & Daniels, 2000, P. 9).
Characteristics of Tannery Waste Water
The effluent from the tannery has some characteristics that is important and must be put into consideration during treatment methods. The wastes from tannery effluent vary in characteristics from one tannery to another. The variation is as result of variation in the structural size of the tannery, the type of chemicals applied in each tannery for a specific process, the quantity of water employed in the setup, and the final product produced in the tannery. The characteristics of the tannery waste is mainly centred on the biochemical oxygen demand, the chemical oxygen Demand, the suspended solids in the waste water, total dissolved solids, and the quantity of chromium and sulphides salts among many (Kongjao et al., 2008, p. 703). The waste from tannery is normally dark brown in color, basic per the PH rating, and contains large organic matter that are a nuisance to the environment if not well disposed. The total COD is around 37,000 mgL-1 while the total Kjeldah Nitrogen is around 273 mg L-1. In addition, N-NH3 and PO43- are averaged at 153 and 21 mg L-1 (Leta et al., 2004, p. 333). The summary of the characteristic are shown in the table below.
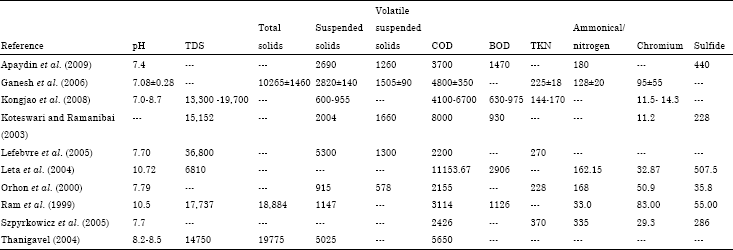
All the values are in mg L-1 accept that of PH.
Management of Tannery Wastes
Tannery waste is the most hazardous refuse on the environment. From the analysis of the characteristics and compositions done above, it was seen that the refuse from tanning industry contains hazardous refuse subsuming metallic components, toxic chemicals, chloride particles, and lime with high dissolved and suspended salts (Uberio, 2003, p. 269). The management process thus involves the laid down procedure that ensures that waste products are well filtered so as to ensure that they do not pollute the environment. The general management process of waste water from tannery takes the form displayed in the diagram bellow. The diagrams include the production process of leather and then the management of the effluent waste from the same.
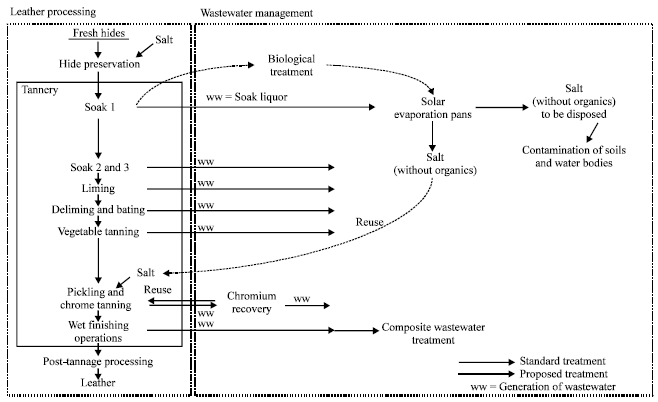
The Available Treatment of Tannery Waste Water
There is a lot of literature existing for the treatment process of waste water form tannery effluent. From the analysis of this existing literature, three methods of treatment of waste water emerge out. They include; biological waste treatment which has been explored by Vijayaraghvan and Murthy (1997, p.151), Wiemann et al. (1998, p.774) and Farabegoli et al. (2004, p.345). There is also chemical waste treatment process that has been explored by Song et al (2004, p. 249). Finally, there is the chemical oxidation waste treatment, which has been handled by many scientist such as Schrank et al. (2003, p. 441) and Sekaran et al. (1996, p. 579).
Application of Chitosan in the Treatment of Tannery Waste Water
The properties of Chitosan make it versatile in many areas. The treatment of tannery waste water has been a subject of intense research for industrial application. As seen in the earlier chapters, waste water for the tannery effluent has a lot of wastes that cannot be removed in one setting. Different mechanisms are applied to the tannery waste treatment for the effectiveness of the process. As seen from the properties of Chitosan, the application for the treatment of tannery waste will be applied in this case. The research for the same will involve aplicateion of Chitosan for a specific process in the tannery waste treatment (Apaydin, Kurt & Gonullu 2009, p.547).
Use of Chitosan for Coagulation and Flocculation of Waste Water from Tannery Industry
Tannery effluent consists of Suspended solids. These are components of insoluble matter found in tannery effluent (waste water. They are problematic to the environment when discharged. They essentially consist of two characteristics. The first one is settleable solids. These are solids with rapid settling rates. They comprises of materials seen in suspension. Settleable solids originate from all stages in leather making process. These solids wastes normally manifest as sludge in waste treatment plant and are normally problematic in the treating process. If this sludge is discharged into water bodies, it will form a blanket in the water bodies and then deprive the aquatic plants and animals from oxygen leading to death (Stoop 2003, p.273). There are also Semi colloidal solids. These are fine solids that cannot settle down even if left for a considerable time. However, they can be filtered form the effluent waste. These solids are obtained from the proteins form the beam house operations. These solids do not directly cause Sludging problems (Ryu, Lee & Chung 2007, p.398). However, when broken down by bacteria over an extended time, they settle down as settleable solids and thus causing Sludging problem when discharged into the river.
The effective removal of these wastes from the tannery effluent requires coagulation and flocculation agent. Flocculation and coagulation process groups the suspended mass of waste in the tannery waste, which are then filtered effectively (Uberoi 2003, p.451).
Coagulation-flocculation Process
This process occurs in two stages giving room for the sedimentation of colloids. Coagulation involves the injection and scattering of chemicals in waste water (Jawahar, et al. 1998, p.672). The flocculation then permits contact between the particles in the waste water that are destabilized. The particles then gather easily to form a visible mass called floc. Floc is then easily eliminated from the waste by decantation process. The process is shown bellow (Rajasimman, Jayakumar, Ravindranath & Chitra 2007, p.154).
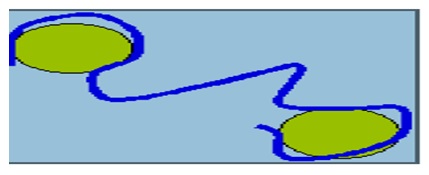
The application of polymer such as Chitosan aids in the bridging of the gap between two far spaced particles. The bridged particles can easily be bound together for the same (Kadam 1990, p.212).
Flocculation and Coagulation Mechanism
The mechanism involved in the formation of floc is essential and forms the point of application of Chitosan in the same. The particles from the tannery waste are in constant motion all the time. This is because these particles have a similar charge. Given that like poles repel one another, the particles will repel each other all the time. The repulsion force in the waste material is responsible for the constant motion of the suspended materials in the tannery waste and thus they cannot settle down or coagulate for effective filtration. In order for the materials to settle down, they must be neutralized by an agent. This agent ought to have protonation capabilities. Chitosan has properties that are vital in the coagulation process. It has the amine group that is responsible for protonation. If applied in the tannery waste as a coagulant, it will react with particles in the tannery waste to form a neutral particle. This is because the polymer (Chitosan) has an ability to donate a proton that will be responsible for the same. The neutralized particles are then combined together by the coagulant. The process is represented by the diagram bellow
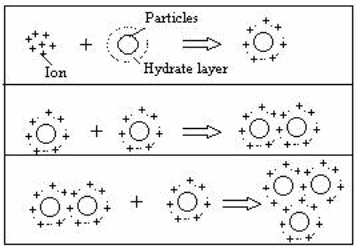
Feasibility of the Process
The use of Chitosan for coagulation process is feasible. Experiments have been carried to determine the feasibility of the process. For the laboratory taste, Bintonite may be used to determine the feasibility of the same. Bintonite has similar characteristic like particles found in the tannery waste. When put in water, it is suspended in the same just like the particles in the waste in the tannery effluent (Verheijen, Weirsema, Hwshoffpol & Dewit 1996, p.65).
The feasibility taste for the same involving the Bintonite suspension with low concentration of 10 mg per litter and high concentration of 300 mg per litter may be used (Sreeram & Ramasami 2003, p.115). When tasted with different dosage of Chitosan, the results are as shown in the graph below.
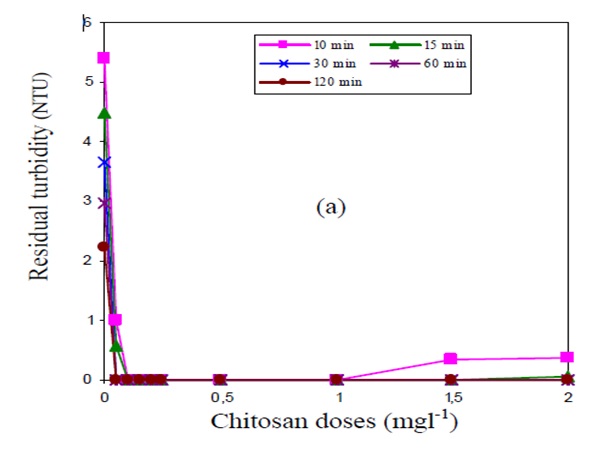
The above result is for the case of low concentration of Bintonite (10mg l-1). For the case of high concentration of Bintonite (300 mg l-1), the results shown bellow is obtained.
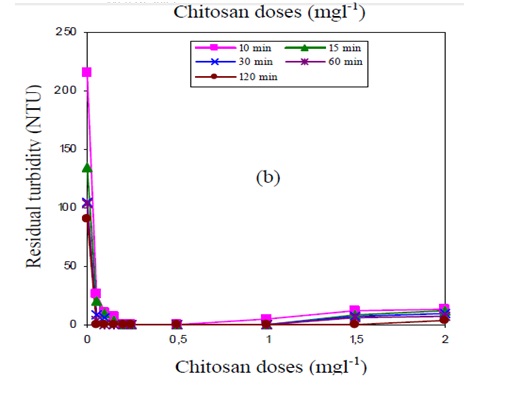
From the information in the above graphs, it can be noted that the graphs represents typical graphs for the coagulation process that is controlled by neutralization of charges (Di Iaconi et al. 2002, p.2209). The particles of Bintonite are anionic in nature. This makes them attracted by the protonated amino groups in Chitosan. This process leads to the neutralization of anionic charges and thus binding together the suspended mater which then settles down rapidly. The turbidity is reached when the total particle are neutralize. Further addition of Chitosan after this point reduces the efficiency of the process (Nandy, Kaul, Shastry, Manivel & Deshpande 1999, p.512).
Use of Chitosan to Remove Fats and Oils and Hairs in Tannery Waste Water
The coagulation ability of Chitosan can be further applied to remove fats and oils from the waste water. The skins and hides have natural oil and grease. During tanning process, the same is released as effluent waste. Also some fatty components are produced when waste waters mingle. Oils on the surface of water blocks oxygen from entering the water and thus can kill the aquatic life. Also, fats on the surface of the waste coagulate to form a ‘mat’ that causes blockage to the environment.
When Chitosan is applied to the waste water from the tannery, it absorbs the fats and oils and forms a gel that floats on the surface that can be easily filtered. According to the research done by the natural health and longevity centre (n.d., p. 3), the effectiveness of Chitosan for the absorption of fats has been proved without doubt. The protonation and decantylation ability of the polymer makes it easy for it to absorb fats, and oils. The experiment involving the same was done on waste water and it revealed that the process was effective as fats were absorbed from the same (Habisch, Jonker, Wegner & Schmidpeter 2005, p.145).
Use of Chitosan for the Removal of Chromium Components in the Tannery Waste Water
Flocculation alone is not sufficient for the treatment of tannery waste. In fact, flocculation is the initial step of treatment of the tannery waste as seen in the treatment process earlier on. The tannery waste has further components that are not easily removed through mechanical means yet they are very dangerous to the environment if not well removed. One such dangerous component is the chromium component (Onyancha et al. 2008, p.614).
Tanning process also yields metallic waste. The waste from metallic components ought to be regarded with care because the wastes from the same are not biodegradable and thus pose the most threat to the environment. There are two forms of chromium metals found in tannery effluent one of them is Chrome 3+ (trivalent chrome, chrome III) (Goltara, Martinez & Mendez 2003, p.210). Although chrome is discharged in soluble form, it undergoes a quick precipitation process when mixed with proteinous waste to form protein-chrome that adds to the sludge. The chromium is further broken down into chromium hydroxide that is stable and persists in the environment as a nuisance to the plants. Even if the chromium remains in soluble form, it is still a nuisance to the environment as it kills some microorganism such as daphnia thus disrupting the food chain. This means that chromium compounds must be eliminated from the tannery effluent (Calheiros, Rangel & Castro 2008, p.873).
Chromium Elimination Using Chitosan
For effective removal of chromium component, an adsorbent must be applied in the same. Chitosan has been found to be an effective adsorbent. In one such experiment, the adsorbent qualities of the Chitosan were observed.
Experimental Design
In order to ascertain the adsorbent quality of Chitosan as applied to the removal of chromium computes, the following procedure is feasible) Sivaprakasam, Mahadevan, Sekar & Rajakumar 2008, p.15).
The chromium stock solution is prepared in accordance to the e-mining water standards with a concentration of 20 mg L-1 (DOE, 2006, p. 4). Deionised water of about 800 ml is then mixed with the chromium. Two percent of the nitric acid is added to the same and the mixture amounts to about 1000 mL (Ram, Bajpai & Parwana 1999). The variation in PH is done with the aid of hydrochloric acid or sodium hydroxide. Several experiment scan be run to maximize the credibility of the results and also to observe the effects of variation of factors such as PH, Chitosan dosage, and contact time. In every experiment, addition of Chitosan into chromium is made and stirred to equilibrium time (Wiegant, Kalker, Sontakke & Zwaag 1999, p.106). After sedimentation, the analysis on the sample of water from the same is done. The removal efficiency is analysed by the formula shown below
E (%) = ( (C0 – C) / C0) ·100
Where
E is the efficiency of chromium absorbent, Co is the initial conentratio of chromiun solution, and C is final chromuim concentration.
According to a similar experiment done by Kabbashi, Abdurahman, Muyibi, and Isam (2009, p. 56), the experimental design was as shown bellow.
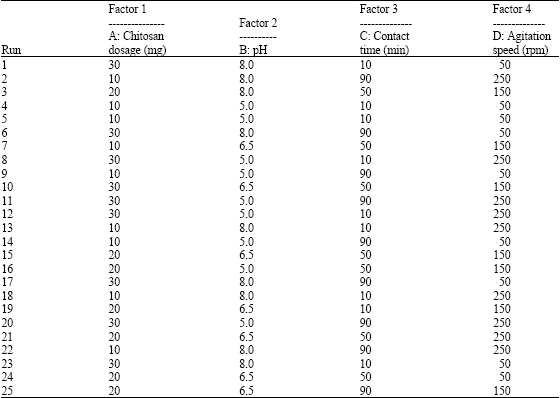
Based on the experimental setup above, the highest removal of chromium was obtained at the 25th run where the efficiency of chromium absorbent was 99% while the lowest was obtained at the 16th run of the experiment. This mean that chromium compounds can be removed from tannery waste using the Chitosan at the optimum condition of 6.5 PH and a constant period of one hour 30 minutes (Thanigavel, 2004). The analysis of the effects of the variables is as shown bellow.
Effects of Chitosan Dosage
Based on the experiment above, it was further ascertained that the further addition of the Chitosan improves the absorption. This can be attested by the diagram below.
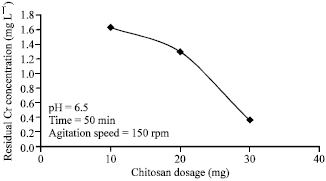
From the graph in the figure above, it is seen that the amount of residual concentration of chromium decrease with the increase in Chitosan dosage. The increased quantity of Chitosan increases the ion exchange sites in the experiment consequently leading to the high amount of adsorption. This means that considerable quantity of Chitosan should be applied in the waste treatment process to enhance the efficiency of the same (Vidal, Nieto, Cooman, Gajardo & Bornhardt 2004, p.211).
The Effect of PH
The PH of the solution plays a big role since the effluents are discharged at a certain PH value. From the experiment above, the PH conditions for the experiment were found to be optimized as shown in the diagram bellow.
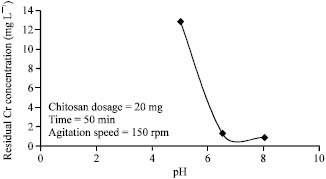
From the graph in the figure above, it is observed that the chromium adsorption is maximum at the PH value of 6.5. According to Tirgar, Golbabaei, Shahtaheri, and Moosavi (2006, p. 306), Chitosan develops solubility problem at PH value of below six by changing into a gel-like substance. This implies that tannery waste, which is normally released at the PH value ranging between 5 and 10, may not be effective for the process. Nevertheless, the process can be modified by the use of an improved form of Chitosan. This is done by coating Chitosan on oil palm shell charcoal (Tirgar et al., 2006, p. 305). The coated Chitosan has several benefits. Apart from making Chitosan to work in a considerable range of PH, it also improves the mass transfer and gel forming behaviour (Rajamani et al. 1995, p.56).
Effects of Contact Time
The effects of contact time on the absorption process are as shown bellow.
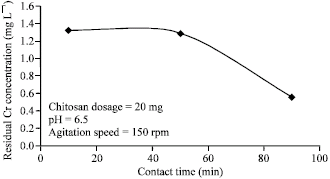
From the graph in the figure above, it is evident that the increase in the residual time leads to the reduction in the residual chromium. This means that the effectiveness of the process increases with the increase in contact time. The industrial application of the process in the tannery waste elimination should include a large waiting tank that is used to hold the residual so as to make the process effective (Genschow, Hegemann & Maschke 1996, p.2075).
Effects of Agitation Speed
Agitation is turning the mixture for the maximum reaction of the process. The results for the same in the experiment are as shown below.
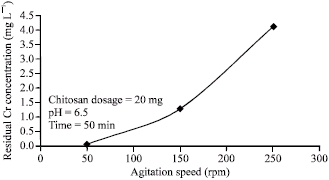
From the figure above, it is seen that the increase in agitation speed increase the residual concentration of chromium. This is attributed to the fact that increased agitation speed improves the speed of the chromium particles movement to the Chitosan. This implies that the industrial application of the same in the tannery waste management should involve agitation process (Eisingerich & Ghardwaj 2011).
Critical Evaluation of the Process
Adsorption refers to the process whereby soluble substances in solution are collected on the surface of the medium interface (Tare, Gupta & Bose 2003, p.21). In the tannery waste water, the chromium is in solution form and thus must be absorbed across the liquid-solid interface. The adsorption process can be physical or chemical (Ganesh, Balaji & Ramanujam 2006, p.1816). For physical adsorption, molecular forces are used to hold one another and hence the process is reversible. For the case of chemical process, the chemical change occurs and thus the process is not reversible (Vankar & Bajpai 2008, p.259). The reaction of Chitosan with metal equilibrium assumes the following equation.
M2+ + RNH2 ← →M (RNH2)2+
The amine group in the Chitosan reacts with the hydrogen ion (H+) as shown bellow
H+ + RNH2 ←→RNH3
From this equation, the amine group has been protonated in acidic media, giving Chitosan a positive charge (Eckenfelder 2002, p.55). This means that the process is not efficient in the acidic media. Under normal conditions, the –NH2 group and the –OH group is used in the same (Munz, Gori, Mori & Lubello 2007, p.356).
The mechanism is represented bellow (Guibal, 2004, p. 38).
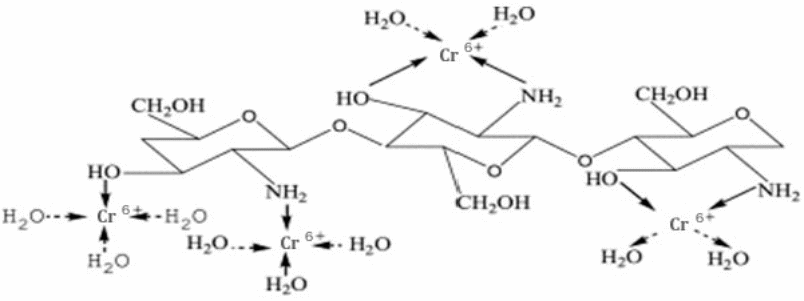
From the above mechanism, the amine group in Chitosan initiates a coordinate bond with the metal ions. The bond forms at the interface of the void orbital of the metals and the free electron pairs of the nitrogen in the amine group.
Application of Chitosan in the Reduction of BOD in Tannery Waste Water
Tannery waste contains microbial which acts on the waste to decompose them into simple products. The decomposition process involves higher consumption of oxygen leading to high competition for oxygen with the aquatic life if the waste was damped into the river. According to the research done by Hemcon (n.d., 2), Chitosan is effective in the reduction of the activities of the bacteria. Chitosan has an antibacterial property whereby it increases the permeability of the cell membranes of the bacteria hence making it release its contents and thus rendering its activity ineffective. The action of Chitosan (in blue) against the cell membrane of bacteria is shown bellow (Hemcon, n.d., p. 3).
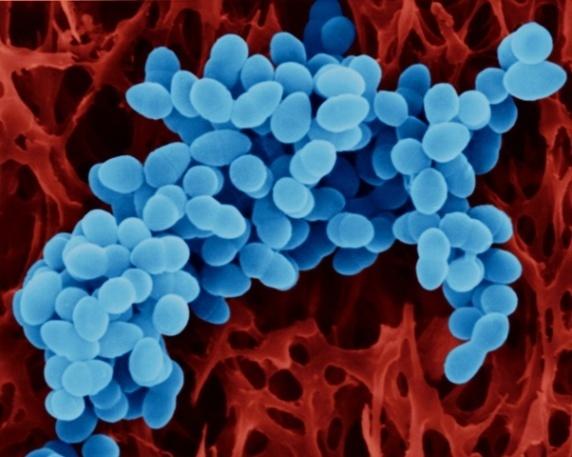
The antibacterial property of Chitosan can be helpful in reducing the BOD and also harmful to the process if not well applied. This is because if all the bacteria are killed, decomposition will not take place (Szpyrkowicz, Kaul & Neti 2005, p.388). However, this is not so because only 99% of the bacterial are eliminate and thus reducing the competition for oxygen in the water. Furthermore, flocculation eliminates ball the organic matter in the tannery waste and thus the work of bacterial will not be desired (Trullyhuge, 2009, p.6).
Advantages of Chitosan That Makes It Feasible For Tannery Waste Treatment
The polymer is available in large quantity. Chitosan is the second largest polymer on earth and thus it can easily be obtained for the application of the many process. Also, the polymer has a god smell. This means that it improves the odour of the tannery effluent on top of treating it. There are no serous side effects. The only side effect is that the Chitosan causes allergies to the people who are allergic to sea creatures. This means that even if it is used in large quantity in the waste treatment, it will not harm other life on earth. The polymer also has a health benefit. It has been found that Chitosan is effective for weight reduction. Thus there are no side effects but rather an added health benefits to the users (Koteswari & Ramanibai 2003, p.163).
Conclusions and Implications
Chitosan is an effective polymer that has many applications. It has been proven that the polymer can be used for the treatment of waste water from tannery effluents. Tannery waste has myriads of components that if not well checked, will be a nuisance to the environment (Jochimsen, Schenk, Jekel & Hegemann 1997, p.211). The existing method of tannery waste treatment does not involve the use of Chitosan; only water treatment involves the use of Chitosan. The properties of Chitosan make it possible for it to be used for the treatment of tannery waste water. The adsorption ability of the polymer makes it suitable for it to be used for Coagulation and Flocculation, removal of chromium compounds and other heavy metals, absorption of fats and oils from the waste, and reduction of BOD and COD of the waste water. The attention of the environmentalists should now shift to the application of Chitosan in the treatment of tannery wastes. The fact that Chitosan is found from natural sea creatures implies that the attention should also focus on the preservation of creatures for the production of Chitosan. If industries will wantonly indulge in the production of Chitosan, the natural source of the polymer will become extinct.
Recommendations
The application of Chitosan in the treatment of tannery waste water should be harnessed by industries dealing in the same. As noted from the properties of Chitosan, it works well in a certain range of PH; 6 to 7.5. These mean that the PH of the tannery waste should be checked before applying Chitosan. Sodium hydroxide or hydrochloric acid should be used for the adjusting the PH of the medium. Also, it was noted that Chitosan has considerable effects on the microorganism. The application of the same should put into consideration that not all microorganisms should be killed by the process (Chung, Choi, Lee & Cho 2004, p.41). Furthermore, Chitosan has no side effects. It is thus the most recommended polymer for the treatment of tannery waste water. In addition, it was found out that the process of chromium removal from the waste takes into consideration the agitation speed and time. This means that the tanneries should set aside a curing tank for the waste. This tank should have an agitator and the waste in it should be held there for a considerable time for effectiveness of the process. Finally, the research into the same is still on and thus anyone is welcome to continue from this point.
List of References
Apaydin, O, Kurt, U & Gonullu, M 2009, ‘An investigation on the tannery wastewater by electro coagulation’, Global NEST Journal, vol.11, pp.546-555.
Ates, E, Orhon, D & Tunay, O 1997, ‘Characterization of tannery wastewater for pretreatment-selected case studies’, Water Sci. Technology, vol.36, pp.217-223.
Banas, J, Plaza, E, Styka, W & Trela, J 1999, ‘SBR technology used for advanced combined municipal and tannery wastewater treatment with high receiving water standards’, Water Sci. Technology, vol.40, pp.451-458.
Banu, J & Kaliappan, S 2007, ‘Treatment of tannery wastewater using hybrid upflow anaerobic sludge blanket reactor’, Journal Environmental Eng. Sci., vol.6, pp.415-421.
Bosnic, M, Buljan J & Daniels, R 2000, ‘Pollutants in tannery effluents united nations industrial development organization’, Regional Programme for Pollution Control in the Tanning Industry in South-East Asia, US/RAS/92/120: pp.1-26.
Calheiros, C, Rangel, A & Castro, P 2008, ‘Evaluation of different substrates to support the growth of Typha latifolia in constructed wetlands treating tannery wastewater over long-term operation’, Bioresour Technol., vol.99, pp.6866-6877.
Chung, Y, Choi, H, Lee, S & Cho, J 2004, ‘Treatment of tannery wastewater with high nitrogen content using anoxic/oxic Membrane Bio-Reactor (MBR)’, Journal of Environ. Sci. Health A., vol.39, pp.1881-1890.
Di Iaconi, C, Lopez, A, Ramadori, R, Di Pinto, A & Passino, R 2002, ‘Combined chemical and biological degradation of tannery wastewater by a periodic submerged filter’, Water Res., vol.36 pp.2205-2214.
Durai, G & Rajasimman, M 2011, ‘Biological treatment of tannery wastewater – a review’, Journal of Environmental Science and Technology, vol.4, pp.1-17.
Eckenfelder, W 2002, Industrial water pollution control, McGraw-Hill, Singapore.
Eisingerich, A & Ghardwaj, G 2011, ‘Corporate social responsibility: does social responsibility help protect a company’s reputation?’ MIT Sloan Management Review, vol. 52, pp.18-19.
Farabegoli, G, Carucci, A, Majone, M & Rolle, E 2004, ‘Biological treatment of tannery wastewater in the presence of chromium’, Journal of Environmental Management, vo.71, pp.345-349.
Ganesh, R, Balaji, G & Ramanujam, R 2006, ‘Biodegradation of tannery wastewater using sequencing batch reactor-respirometric assessment’, Bioresour Technology, vol.97, pp.1815-1821.
Genschow, E, Hegemann, W & Maschke, C 1996, ‘Biological sulfate removal from tannery wastewater in a two-stage anaerobic treatment’, Water Res., vol. 30, pp.2072-2078.
Goltara, A, Martinez, J & Mendez, R 2003, ‘Carbon and nitrogen removal from tannery wastewater with a membrane bioreactor’, Water Sci. Technol., vol. 48, pp. 207-214.
Goosen, M 1996, Applications of chitin and chitosan, CRC Press, London.
Guibal, E 2004, ‘Interactions of metal ions with Chitosan-based sorbents: A review’. Separation and Purification Technology, vol.38, pp.43-74.
Habisch, A, Jonker, j, Wegner, M & Schmidpeter, R 2005, Corporate social responsibility across the Europe, Springer, Heidelberg.
Haydar, S, Aziz, J & Ahmad, M 2007, ‘Biological treatment of tannery wastewater using activated sludge processes, Pak. J. Eng. Applied Sci., vol.1, pp.61-66.
HemCon, n.d., Antibacterial properties of Chitosan, Web.
Jawahar, A, Chinnadurai, M, Ponselvan, J & Annadurai, G 1998, ‘Pollution from tanneries and options for treatment of effluent’, Ind. J. Environ. Protec., vol.18, pp.672-672.
Jochimsen, J, Schenk, H, Jekel, M & Hegemann, W 1997, ‘Combined oxidative and biological treatment for separated streams of tannery wastewater’, Water Sci. Technol., vol.36, pp.209-216.
Kadam, R 1990, ‘Treatment of tannery wastes’, Ind. J. Environ. Protec., vol.10, pp. 212-212.
Khan, S, Kawaja, M, Khan, M, Ghani, H & Kazmi, S 1999, ‘Environmental impacts and mitigation costs associated with cloth and leather exports from Pakistan’, A Report on Trade and Sustainable development Submitted by Sustainable Development Policy Institute and IUCNP to IISD Canada for the IISD/IUCN/IDRC.
Kongjao, S, Damronglerd, S & Hunsom, M 2008, ‘Simultaneous removal of organic and inorganic pollutants in tannery wastewater using electrocoagulation technique’, Korean J. Chem. Eng., vol.25, pp.703-709.
Koteswari, Y & Ramanibai, R 2003, ‘The effect of tannery effluent on the colonization rate of plankets: A microcosm study’, Turk. J. Biol., vol.27, pp.163-170.
Lefebvre, O, Vasudevan, N, Torrijos, M, Thanasekaran, K & Moletta, R 2005, ‘Halophilic biological treatment of tannery soaks liquor in a sequencing batch reactor’, Water Res., vol.39, pp.1471-1480.
Lefebvre, O, Vasudevan, N, Torrijos, M, Thanasekaran, K & Moletta, R 2006, ‘Anaerobic digestion of tannery soak liquor with an aerobic post-treatment’, Water Res., vol.40, pp.1492-1500.
Leta, S, Assefa, F, Gumaelius, L & Dalhammar, G 2004, ‘Biological nitrogen and organic matter removal from tannery wastewater in pilot plant operations in Ethiopia’, Applied Microbiol. Biotechnol, vol.66, pp.333-339.
Mandal, T, Maity, S, Dasgupta, D & Datta, S 2010, ‘Advanced oxidation process and biotreatment: Their roles in combined industrial wastewater treatment’, Desalination, vol.250, pp.87-94.
Mazumder, D, Mukherjee, S & Ray, P 2008, ‘Treatment of tannery wastewater in a hybrid biological reactor’, Int. J. Environ. Pollut, vol.34, pp.43-56.
Milstein, M 2003, ‘Creating sustainable value’, Academy of Management Executive, vol.17, no.2, pp.56-67
Munz, G, De Angelis, D, Gori, R, Mori, G, Casarci, M & Lubello, C 2009, ‘The role of tannins in conventional and membrane treatment of tannery wastewater’, J. Hazard. Mater., vol.164, pp.733-739.
Munz, G, Gori, R, Mori, G & Lubello, C 2007, ‘Powdered activated carbon and membrane bioreactors (MBRPAC) for tannery wastewater treatment: Long term effect on biological and filtration process performances’, Desalination, vol.207, pp.349-360.
Murugesan, V & Elangoan, R 1994, ‘Biokinetic parameters for activated sludge process treating vegetable tannery waste’, Ind. J. Environ. Protec., vol.14, pp. 511-515.
Nandy, T, Kaul, S, Shastry, S, Manivel, W & Deshpande, C 1999, ‘Waste-water management in cluster of tanneries in Tamilnadu through implementation of common treatment plants’, J. Sci. Ind. Res., vol.58, pp.475-516.
Onyancha, D, Mavura, W, Ngila, J, Ongoma, P & Chacha, J 2008, ‘Studies of chromium removal from tannery wastewaters by algae biosorbents, Spirogyra condensate and Rhizocolonium hieroglyphicum’, J. Hazard. Mater, vol.158, pp.605-614.
Orhon, D, Ates, E & Sozen, S 2000, ‘Experimental evaluation of the nitrification kinetics for tannery wastewaters’, Water SA, vol.26, pp.43-52.
Rajamani, S, Ramasami, T, Langerwerf, JS & Schappman, JE 1995, ‘Environmental management in tanneries-feasible chromium recovery and reuse system’, Proceedings of the 3rd International Conference on Appropriate Waste Management Technologies for Developing Countries, (AWMTDC95), Nagpur, India, pp.965-969.
Rajamani, S, Suthathrarajan, R, Ravindranath, E, Mulder, A, Groenestijn, J & Langerwerf, S 1997, ‘Treatment of tannery wastewater using Upflow Anaerobic Sludge Blanket (UASB) system’, Proceedings of the 31st Leather Research Industry Get-Together, (LRIGT97), Central Leather Research Institute, Adyar, India, pp.57-57.
Rajasimman, M, Jayakumar, M, Ravindranath, E & Chitra, K 2007, ‘Treatment of solid and liquid wastes from tanneries in an UASB reactor’, Proceedings of the 60th Annual Session of Indian Institute of Chemical Engineers, CHEMCON-2007, Kolkatta, India.
Ram, B, Bajpai, P & Parwana, K 1999, ‘Kinetics of chrome-tannery effluent treatment by the Activated sludge system’, Process Biochem, vol.35, pp.255-265.
Ramteke, P, Awasthi, S, Srinath, T & Joseph, B 2010, ‘Efficiency assessment of Common Effluent Treatment Plant (CETP) treating tannery effluents’, Environ. Monitor. Assessment, vol.10, no.1007, pp.1149-1156.
Ryu, H, Lee, S & Chung, K 2007, ‘Chemical oxygen demand removal efficiency of biological treatment process treating tannery wastewater following seawater flocculation’, Environ. Eng., vol.24, pp.394-399.
Schrank, S, Jos, HJ, Moreira, RF & Schroder, HF 2003, ‘Fentons oxidation of various-based tanning materials’, Desalination, vol.50, pp.411-423.
Sekaran, G, Chitra, K, Mariappan, M & Raghavan, K 1996, ‘Removal of sulphide in anaerobically treated tannery wastewater by wet air oxidation’, J. Environ. Sci. Health A., vol.31, pp.579-598.
Shakoori, A, Makhdoom, M & Haq, U 2000, ‘Hexavalent chromium reduction by a dichromate-resistant gram-positive bacterium isolated from effluents of tanneries’, Applied Microbiol. Biotechnology, vol.53, pp.348-351.
Sivaprakasam, S, Mahadevan, S, Sekar, S & Rajakumar, S 2008, ‘Biological treatment of tannery wastewater by using salt-tolerant bacterial strains’, Microbial. Cell Fact., vol.7, pp.15-15.
Song, Z, Williams, C & Edyvean, RG 2003, ‘Tannery wastewater treatment using an Upflow Anaerobic Fixed Biofilm Reactor (UAFBR)’, Environ. Eng. Sci., vol.20, pp.587-599.
Song, Z, Williams, C & Edyvean, RG 2004, ‘Treatment of tannery wastewater by chemical coagulation,’ Desalination, vol.164, pp.249-259.
Sreeram, K & Ramasami, T 2003, ‘Sustaining tanning process through conservation, recovery and better utilization of chromium,’ Resource Conserv. Recycling, vol.38, pp.185-212.
Srivastava, J, Chandra, H, Tripathi, K, Naraian, R & Sahu, KR 2008, ‘Removal of chromium (VI) through biosorption by the Pseudomonas spp. isolated from tannery effluent, Journal of Basic Microbiology, vol.48, pp.135-139.
Srivastava, S & Thakur, I 2006, ‘Isolation and process parameter optimization of Aspergillus sp. for removal of chromium from tannery effluent’, Bioresour. Technol., vol.97, pp.1167-1173.
Srivastava, S, Ahmad, H & Thakur, I 2007, ‘Removal of chromium and pentachlorophenol from tannery effluents,’ Biores. Technol., vol. 98, pp.1128-1132.
Stoop, ML 2003, ‘Water management of production systems optimized by environmentally oriented integral chain management: Case study of leather manufacturing in developing countries,’ Technovation, vol.23, pp.265-278.
Sumathi, KM, Mahimairaja, S & Naidu, R 2005, ‘Use of low-cost biological wastes and vermiculite for removal of chromium from tannery effluent’, Bioresour. Technol., vol.96, pp.309-316.
Szpyrkowicz, L, Kaul, SN & Neti, RN 2005, ‘Tannery wastewater treatment by electro-oxidation coupled with a biological process,’ Journal Applied Electrochemistry, vol.35, pp.381-390.
Szpyrkowicz, L, Stern, SR & Grandi, FZ 1991, ‘Nitrification and denitrification of tannery wastewaters,’ Water Res., vol.25, pp.1351-1356.
Tare, V, Gupta, S & Bose, P 2003, ‘Case studies on biological treatment of tannery effluents in India,’ Journal of Air Waste Manage. Assoc., vol.53, pp.976-982.
Thanigavel, M 2004, ‘Biodegradation of tannery effluent in fluidized bed bioreactor with low density biomass support’, M.Tech. Thesis, Annamalai University, Tamilnadu, India.
Trullyhuge, 2009, Chitosan Benefits, Web.
Uberoi, NK 2003, Environmental management, Excel Books Publisher, New Delhi,
Vankar, P & Bajpai, D 2008, ‘Phyto-remediation of chrome-VI of tannery effluent by Trichoderma species,’ Desalination, vol. 222, pp. 255-262.
Verheijen, LA, Weirsema, D, Hwshoffpol, LW & Dewit, J 1996, Live stock and the environment: finding a balance management of waste from animal product processing, International Agriculture Centre, Wageningen.
Vidal, G, Nieto, J, Cooman, K, Gajardo, M & Bornhardt, C 2004, ‘Unhairing effluents treated by an activated sludge system,’ Journal Hazard. Mater, vol.112, pp.143-149.
Vijayaraghvan, K & Murthy, DV 1997, ‘Effect of toxic substances in anaerobic treatment of tannery wastewater’, Bioprocess Biosyst. Eng., vol.16, pp.151-155.
Wang, YS, Pan, ZY, Lang, J, Xu, M & Zheng, Y 2007, ‘Bioleaching of chromium from tannery sludge by indigenous, Acidthiobacillus thiooxidans,’ Journal Hazard. Mater., vol.147, pp.319-324.
Wiegant, W, Kalker, T, Sontakke, V & Zwaag, R 1999, ‘Full scale experience with tannery water management: An integrated approach,’ Water Sci. Technol., vol.39, pp.169-176.
Wiemann, M, Schenk, H & Hegemann, W 1998, ‘Anaerobic treatment of tannery wastewater with simultaneous sulphide elimination,’ Water Res., vol.32, pp.774-780.
Yuan, Z 2007, ‘Study on the synthesis and catalyst oxidation properties of chitosan bound nickel (II) complexes,’ Chemical Industry Times, vol.21, no.5, pp.22-24.