The setting for this project is a primary community clinic situated in Seattle. It serves the most part of the population living in this urban area and is visited by many patients seeking primary care for their chronic conditions. The clinic’s personnel have noticed that older patients’ non-adherence often stems from their false beliefs as to the management of hypertension and chronic conditions in general. False beliefs are a common problem among hypertensive patients who frequently believe that they should take their medications only when they feel ill (Chan & Horne, 2018). Therefore, patient education is a justified intervention for increasing adherence to hypertensive drugs in this case.
In order to get access to potential subjects, leaflets will be placed in the lobby and the rooms of the clinic. Physicians and nurses will also be provided with leaflets and asked to give them to patients eligible to participate in the study. There may be ethical objections to the experimental design of the study that aims at assessing the impact of increased adherence as a result of adherence intervention (Zullig et al., 2018). Therefore, before recruiting potential subjects, it will be necessary for them to fill the consent form (see Appendix A).
Timeline
As identified in the PICOT question, the time frame for this project will be six months for delivering the intervention and assessing its outcomes. One additional month will be required for the arrangement of the study, which will include discussions of the project with stakeholders, training of the involved staff, and the recruitment of the participants. A detailed timeline for this project is included in Appendix B.
Solution Process and Resources
In order to implement the proposed solution, specific process changes should be introduced. The patient care in the clinic should become more patient-centered so that patients were confident that healthcare professionals were interested in offering them the best treatment option. Physicians involved in this project will partly rely on patients’ honesty and conscientiousness, so it is essential to establish trust between healthcare professionals and patients to encourage patients’ adherence.
The proposed solution will require human and financial resources. Human resources will include physicians and nurses who will provide education on managing hypertension to patients. Such community stakeholders, as patients’ families, also can be included in human resources since they can contribute to patients’ adherence. Financial resources will include money required for printing leaflets and payroll costs for additional working hours spent by healthcare professionals on the participation in the study. The resources required for this project are listed in Appendix C.
Solution Methods and Instruments
The process of delivering the intervention will be patient education sessions. It is planned to have three group sessions in the second month of the project after the participants are recruited and consented to participate in the study. Patient education should include informing patients about risks associated with non-adherence to antihypertensive drugs and the importance of medication adherence for slowing disease progression (Kini & Ho, 2018). Patients should also be educated during their regular visits to their physicians to make sure they realize the peculiarity of choric disease management. Training for the staff will be necessary to teach physicians and nurses to engage in more patient-centered communication and encourage patients’ adherence.
The methods and instruments to be used for monitoring the implementation of the solution will include interviews with patients and urinary drug tests. Interviews will be intended to assess adherence and identify barriers to it and should be conducted in a collaborative communication style to establish trust (Burnier & Egan, 2019). The examples of questions to be used in interviews are listed in Appendix D. In order to increase the precision of patients’ adherence measurement, urine drug tests should be performed.
Data Collection and Management
The data collection will be performed by means of the Hill-Bone Compliance to High Blood Pressure Therapy Scale (see Appendix E). This scale includes 14 items covering three areas of hypertension therapy, such as medication regimens, diet, and medical appointment keeping (Etebari et al., 2019). It is a common tool for assessing patients’ adherence to antihypertensive drugs, and it is useful for this project since it can be performed during interviews described in the previous section. Data analysis will be performed by calculating patients’ scores, which will be interpreted as the level of patients’ adherence to medications. Data management will be performed by an assigned person who will collect patient data gathered by different physicians.
Budget
The project does not use any specific equipment and does not require traveling, so these items are absent from the budget plan (see Appendix F). Payroll costs include assumed additional hours that physicians and nurses will have to spend on educating patients. The costs for urinary tests were calculated, assuming there would be 15 participants taking tests two times per project. Leaflets will be necessary for recruiting participants, and materials and supplies will be necessary for healthcare professionals to conduct interviews and questionnaires. Finally, the training conducted by the project coordinator will be necessary to educate the staff on adherence interventions.
Maintenance, Adjustment, and Discontinuation of the Solution
The project will be maintained by inviting the participants to the community clinic to be interviewed and educated. During these visits, physicians will need to identify barriers to adherence, such as forgetfulness. To address these barriers, physicians may suggest reminding patients to take their drugs by phone calls or text messages (Kini & Ho, 2018). To extend the solution, physicians may involve pharmacists to encourage patients’ adherence. Upon the completion of the project, data will be analyzed and summarized, and patients will be able to obtain their results upon request.
References
Burnier, M., & Egan, B. M. (2019). Adherence in hypertension. Circulation Research, 124(7), 1124-1140.
Chan, A., & Horne, R. (2018). Beliefs and adherence in hypertension and cardiovascular protection. In M. Burnier (Ed.), Drug adherence in hypertension and cardiovascular protection (pp. 123-141). Springer.
Etebari, F., Pezeshki, M. Z., & Fakour, S. (2019). Factors related to the non-adherence of medication and nonpharmacological recommendations in high blood pressure patients. Journal of Cardiovascular and Thoracic Research, 11(1), 28-34.
Kini, V., & Ho, P. M. (2018). Interventions to improve medication adherence. JAMA, 320(23), 2461-2473.
Zullig, L., Deschodt, M., Liska, J., Bosworth, H. B., & De Geest, S. (2018). Moving from the trial to the real world: Improving medication adherence using insights of implementation science. Annual Review of Pharmacology and Toxicology, 59(1), 1-23.
Appendix A
Study Information & Consent Form
The purpose of this research is to study the effect of adherence to antihypertensive drugs compared to medication non-adherence on a decrease in hypertension events.
If you participate in this research, you will be asked to provide your demographic information, attend educational sessions at the community clinic, and follow the instructions given be the physician. You will be asked to comply with your antihypertensive regimen and honestly report your medication adherence to the physician. You will also be asked to undergo a urine drug test to enhance the precision of the research results.
It is possible you may experience some discomfort during or as a result of your participation in this study. If you experience emotional discomfort as a result of your participation, you are encouraged to visit the Counseling Center (to make an appointment, call 555-5555).
There will be no personal benefits to you from your participation in this research. However, the results of the research may contribute to advances in the field of hypertension management. The duration of this research project is six months.
Your participation in this research is strictly voluntary. You may refuse to participate at all, or choose to stop your participation at any point in the research without fear of penalty or negative consequence.
The information/data you provide for this research will be treated confidentially, and all raw data will be kept in a secured file by the researcher. Results of the research will be reported as aggregate summary data only, and no individually identifiable information will be presented unless explicit permission is given to do so.
You also have the right to review the results of the research if you wish to do so. A copy of the results may be obtained by contacting the researcher: [Researcher name and contact information]
If further questions arise, or you feel you have been treated unfairly, please contact Dr. Kate Clark, chair of the Eastern Mennonite University Institutional Review Board, Eastern Mennonite University, 1200 Park Rd., Harrisonburg, VA, ph. (540) 432-4710, email: [email protected].
Participant consent
I, (print full name), have read and understand the foregoing information explaining the purpose of this research and my rights and responsibilities as a subject. My signature below designates my consent to participate in this research, according to the terms and conditions listed above.
- Signature
- Date
Appendix B
Timeline
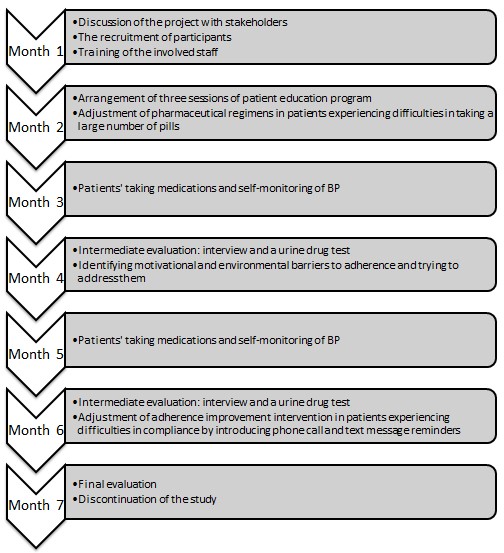
Appendix C
The Resource List
Human Resources:
- Physicians and nurses: patient education and adherence assessment.
- Patient families: improving patients’ adherence.
Financial Resources:
- Money for printing leaflets
- Finances for healthcare professionals’ payroll costs
Appendix D
Monitoring Instruments: Possible Interview Questions
An interview with a patient may include the following questions to ensure patient-centered communication:
- Are you having any problems with your medications such as they’re too costly or cause unpleasant adverse effects?
- How does a low-salt diet affect you?
- What are some of the difficulties you have with a low-salt diet?
- What makes it difficult for you to take your medications?
- Tell me what you know about high BP.
- Thinking about what we talked about today, what is one thing you can do differently this week?
(Source: Burnier & Egan, 2019)
Appendix E
Data Collection Tool
The Hill-Bone Compliance to High Blood Pressure Therapy Scale:
- How often do you forget to take your HBP medicine?
- How often do you decide not to take your HBP medicine?
- How often do you eat salty food?
- How often do you shake salt on your food before you eat it?
- How often do you eat fast food?
- How often do you make the next appointment before you leave the doctor’s office?
- How often do you miss scheduled appointments?
- How often do you forget to get prescriptions filled?
- How often do you run out of HBP pills?
- How often do you skip your HBP medicine before you go to the doctor?
- How often do you miss taking your HBP pills when you feel better?
- How often do you miss taking your HBP pills when you feel sick?
- How often do you take someone else’s HBP pills?
- How often do you miss taking your HBP pills when you are careless?
Possible answers:
- None of the time
- Some of the time
- Most of the time
- All of the time
Appendix F
The Budget Plan
Table 1. The budget plan for the project.