Executive Summary
Flame test and chemical fingerprinting are analytical procedures that are used to identify metals or metalloid compounds. When subjected to heat, metals emit specific electromagnetic wavelengths, thereby, a particular color of light. The results of this experiment show that lithium gives off a red flame, sodium an orange flame, potassium with cobalt glass emits a violet flame, potassium with cobalt glass gives off a purple-red flame, calcium an orange-red flame, strontium a red flame, and lastly barium a pale green flame. Furthermore, the flame test’s findings can be complemented by the chemical fingerprinting analysis that uses the principle of precipitate formation to identify a metal.
Introduction
Under normal conditions, the atoms or ions of an element exist in the ground state. In this state, of stable energy, the electrons occupy the lowest energy levels available. However, when subjected to high temperatures, the atoms or ions absorb energy and “jump” to higher energy levels, thus, reaching an excited state. This new configuration is unstable; therefore, the atoms quickly “fall” back to the initial ground state that is energetically more favorable. As they return to their normal levels, the energy which was absorbed is emitted in the form of electromagnetic energy, and this might be in the form of light. Electromagnetic radiation comprises perpendicular waves oscillating in magnetic or electric fields either through space or through matter. These waves travel at the speed of light, c, which is quantitated as 2.998 × 108 m/s. The relationship between the frequency, wavelength and speed of an electromagnetic wave is represented by the following equation: c = λ x f, where λ the wavelength, and f is the frequency
The difference in the radiation between the stable and excited state adheres to the Planck’s Law:
E photon = h × f, where h is the Planck’s constant, with a value of 6.626 × 10-34
Although the excited state is peculiar for each element, the ground state is always the same. As a result, the radiation emitted is unique for each item, enabling the identification of the elements. Visible light is the most familiar form of electromagnetic radiation, with the variation in the wavelengths manifested in different colors.

Currently, the flame test is an analytic technique that is used to identify and quantify this principle. In this test, when the emitted photons fall in the visible region of the spectrum, they might be perceived as lines of different colors, which can be referred to as the line emission spectrum. This allows for fingerprinting of the element. The identity of an element can be further confirmed by chemical fingerprinting. Some cations form relatively insoluble salts that precipitate out of aqueous solutions. For example, most sulfate salts are soluble except those of barium, lead, mercury, and calcium.
Materials and Equipment
- 0.50 M solutions of LiCl, NaCl, KCl, CaCl2, SrCl2, and BaCl2
- A Bunsen burner
- Wooden splints
- A striker
- Cobalt glass plates
- Well-plates
- 0.10 M solution of (NH4)2CO3, (NH4)2SO4 and 0.1(NH4)2HPO4
- Beaker (250 to 500 mL) half-filled with tap water
- Unknown solution
- Beral pipette
Method
Flame Test
In this test, a wooden splint is dipped into 0.5M LiCl solution, and then held over a Bunsen burner flame. The dominant color flame is observed and recorded. This process is repeated for the remaining five solutions (NaCl, KCl, CaCl2, SrCl2, and BaCl2) in a sequential manner using a fresh wooden splint each time.
Chemical Fingerprinting
Using a Beral pipette, add 0.10 M solutions of (NH4)2CO3, (NH4)2SO4 and 0.1(NH4)2HPO4 to three plates containing NaCl. Observe and record as to whether a colorless solution or a precipitate was formed due to the mixing of the two solutions. Repeat this for KCl, CaCl2, SrCl2, and BaCl2.
Results
Based on the experimental lab videos, the objective of the experiment was to determine the identity or possible identity of a metal ion present in an ionic compound. When a compound is burnt on a flame, a characteristic color visible to the naked eye is emitted, and this was observed to vary between ionic solutions (as in Tables 1 and 2). Furthermore, the identity of the metals was further narrowed upon through the chemical fingerprinting technique. This is because metals have different solubility in various solutions. A Bunsen burner, cobalt blue glass wooden splints, and 0.5M solutions of LiCl, NaCl, KCl, CaCl2, SrCl2, and BaCl2 were used to facilitate the Flame test. On the other hand, the before-mentioned solutions were added to 0.10 M solution of (NH4)2CO3, (NH4)2SO4, and 0.1(NH4)2HPO4 each, to allow for chemical fingerprinting.
Tables 1 and 2 show the results of the experiment. In Table 1, different metals emitted distinctive colors, and this is attributed to the characteristic electromagnetic wavelengths that were given off when they were burnt. Overall, the experiment was good; however, I believe that the cobalt blue glass should have also been used with other elements other than potassium to enhance the accuracy of results. I have learned that there are more simplified methods of fingerprinting cations instead of using sophisticated equipment such as spectrometers.
Questions
Pre-Lab Questions
- When absorbing energy, the electron moves from the ground state to an excited state.
- When an electron releases energy, it moves from an excited state to the ground state.
- When electrons absorb energy, they transition from lower to higher energy levels. The energy absorbed might be in the form of heat as in the case of the flame test. As the electrons return to the lower energy levels, the ground state, energy is released often in the form of electromagnetic radiation.
- Chloride salts are used as they are easily volatized by the flame.
- Lithium.
Post-Lab Questions
- It possesses an optical filter characteristic that enables it to filter out by absorbing the yellow flame emitted by traces of sodium. Therefore, this allows for the easy visualization of other colors within a flame.
- A yellow-green flame suggests the presence of the Barium cations (Ba+). Barium’s carbonate, phosphate and sulfate compounds form precipitates.
- Electronic configuration of each metal investigated in this module:
- Li – 2.1
- Na – 2.8.1
- K – 2.8.8.1
- Ca – 2.8.8.2
- Sr – 2.8.8.8.2
- Ba – 2.8.8.8.8.2
The electron configuration of each metal predicts the reaction outcome observed in chemical fingerprinting. This is because the solubility of sulfates, phosphates and carbonates decreases with an increase in the number of electrons.
- Fireworks root back to China in the 6th Century during the Sung Dynasty (Grush, 2015). An exploding firework often undergoes several chemical reactions that occur either simultaneously on in rapid sequence. The four primary chemical ingredients include colorants, fuel, oxidizer, binder, and a chlorine donor. Fireworks require plenty of oxygen to facilitate burning, and this is where oxidizers come in. The most commonly used oxidizers are nitrates, perchlorates, and chlorates. Furthermore, they require fuel, in which typically, the gunpowder composition comprising potassium nitrate, sulfur, and charcoal are utilized. The oxidizer and fuel allow for the lighting of the tiny rocket from the wick to the main body, thereby propelling it to the sky. Also included in the capsule are color producers (metal salts, such as Strontium, Barium, Copper, Calcium, Sodium, and Magnesium, among others), which emit specific wavelengths of visible light when burnt and a binder that holds the capsule mixture together. The binder is usually in the form of a starch known as dextrin that is dampened with water. For red, a strontium compound is required; for green, a barium compound is needed. Copper produces blue colors, and sodium compounds result in yellow colors.
- Different atoms/ions emit different colors of light because they produce different wavelengths of electromagnetic radiation.
- Apart from fireworks, other examples of colorful light emissions include neon signs and street lights. Neon signs emit light when an electric current passes through neon gas. This results in the excitation of electrons.
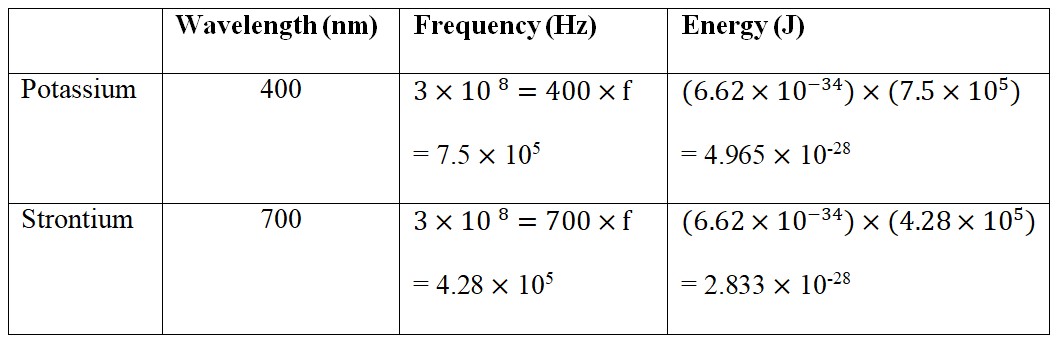
- Potassium and Strontium light emissions.
Conclusion
This experiment aimed to identify cations based on the colors they emit when subjected to heat. Each type of cation was associated with a distinct flame; moreover, the cobalt blue glass can be used as an optical filter to remove the yellow flame given off by sodium. Therefore, this improves the accuracy of the flame test. Besides, the findings of the flame test can be further confirmed by chemical fingerprinting in which particular metal salts form precipitates when reacted with sulfates, phosphates, and carbonates.
Reference
Grush, L. (2015). The chemistry behind a firework explosion. The Verge. Web.