Introduction
Microbiological examination of samples is a qualitative tool for their identification. Moreover, with the help of developed methods of laboratory analysis, it becomes possible not only to determine the belonging of a microorganism to a particular taxon but also to establish the strain identification of the sample in the shortest possible time. It follows that microbiology answers questions of qualitative analysis and identification of biochemical properties of the observed sample. In turn, such capabilities have a high scientific potential for laboratory and clinical research since the possible pathogenicity of the prokaryote to the human organism can be determined in advance. Thus, the overall goal of this work is to summarize the available data on the microbiological identification of an unknown specimen.
In the case study described, a preparation with an unknown microorganism turns out to be isolated under clinical conditions by a laboratory technician. Because the worker lacked the competence to identify it himself, he decided to contact the author. The advice that was given was a general conceptualization of how to identify microorganisms depending on their structure, the tests used, and the detailed procedure that a laboratory technician needs to implement for a successful microbiological analysis. The result of this report was the detection of a strain isolated in the clinic: it was Pseudomonas aeruginosa, a common Gram-negative pathogenic bacillus, which initiates nosocomial infections.
Materials and Methods
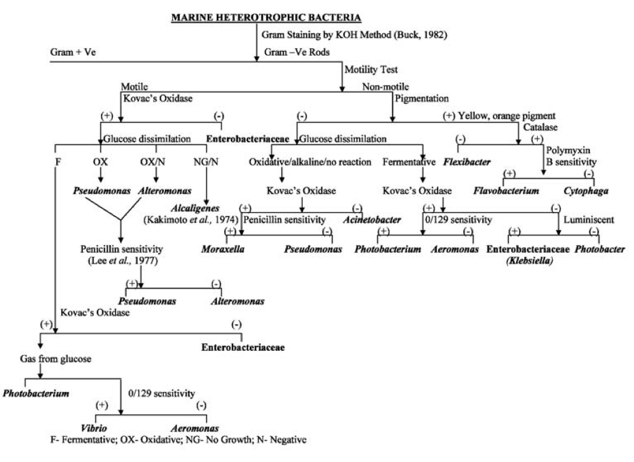
Qualitative identification of the unknown microorganism required a series of sequential laboratory tests, narrowing the range of potential candidates: this was achieved using the flowchart illustrated in figure 1. Thus, the first step was the Gram staining test: for this purpose, crystal violet, iodine etchant, decolorizer (alcohol), and safranin were applied sequentially to the sample. Gram-negative bacteria were stained red, while gram-positive bacteria were stained purple. The microbiological analysis then required examination of the sample with a microscope to determine the morphology. The third step was to test the ability to self-ferment glucose: for this purpose, the sample preheated to room temperature was seeded with purple glucose broth, and a control tube with the broth was created in parallel. Cultivation took place for 3-5 days at 35-37℃: if the tube ended up with a yellow stain, this indicated a positive reaction to the GF test.
Next, an oxidative test was performed, the results of which would establish the ability of the organism to produce cytochrome oxidase. Kovacs’, Gordon and McLeod’s, and Gaby and Hadley’s reagents were used for this purpose: the appearance of purple staining on a cotton swab previously moistened with the three reagents and touched to the colony indicated a positive reaction. Finally, it was appropriate to perform cultivation tests that would yield results regarding the optimal temperature for colony formation, its pigmentation, and the type of agar used for cultivation.
Results
For this microbiological analysis, the flowchart proposed in the Methods section was used. The flowchart was shown to satisfy the experimental conditions perfectly, as the end result of the identification was the accurate identification of the microorganism contained in the isolated colony. Examination with an optical microscope showed a bacilliform morphology of the motile pathogen, with the overall coloration of the colony at the time of culturing without Gram staining being blue-green-yellow. The Gram test showed that the microorganism after treatment with safranin was reddish in color, which means that the pathogen belonged to the domain of Gram-negative bacteria.
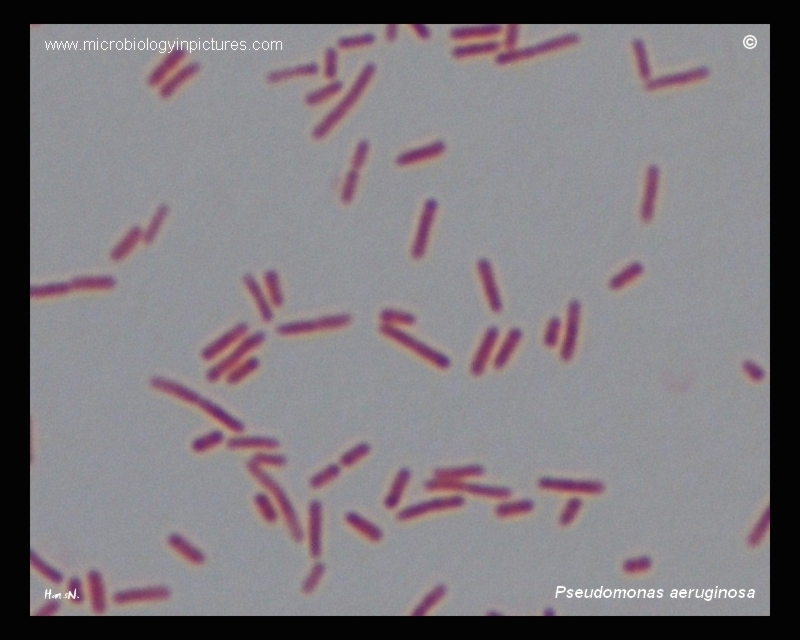
A series of enzymatic tests were aimed at critically examining the biochemical properties of the pathogen’s metabolism. Seeding with the GF test showed the absence of any staining, indicating an absence of reaction to this carbohydrate. The oxidative test gave purple staining of the cotton swab (smear method was used): consequently, the unknown organism was prone to cytochrome oxidase production, which is characteristic of most aerobic prokaryotes. As a consequence, in combination with the cultivation test, it was concluded that the unknown strain was Pseudomonas aeruginosa. Below is a summary table of the results summarizing the data obtained in each step.
Table 1. Summary results of all qualitative microbiological analysis tests.
Discussion
The microbiological assay is a useful clinical tool for the qualitative identification of an unknown organism. The advantages of such assays include a wide variety of methods and tests that can be used to examine the subject properties of the organism. More specifically, the metabolic ability of a strain to produce a particular carbohydrate or protein can be tested with individual tests, which means that modern microbiology can thoroughly investigate an unknown organism. In the present work, such a sample was the pathogenic strain of the bacterium Pseudomonas aeruginosa, known primarily as the causative agent of nosocomial infections. First of all, we should recognize the expectation of such a result since the pathogen isolated in clinical conditions is almost certainly Pseudomonas aeruginosa, which is widespread there. The natural conditions of the prokaryote are aqueous media, soil, and clinical non-sterile facilities. Entry of the pathogen into the body is due to purulent infections and patient abscesses, with the presence of chronic cystitis and enteritis being aggravating factors (CDC, 2017). In addition, an individual’s weakened immune system also makes it easier for the pathogen to take hold inside. When Pseudomonas aeruginosa enters the body, the developmental phase of the bacterium is initiated, which is realized through infection of the patient’s blood and lungs. Drug treatment is complicated by the high antibiotic resistance of the pathogen due to its presence in the clinical setting. As a consequence, only the development of new antibiotic drugs is the right solution for the therapeutic treatment of the infection.
This microbiological assay has a high degree of accuracy and reliability, but it does not take into account the general metabolic factors of the pathogen. For more sensitive laboratory testing, a relevant strategy would be to use the full range of biochemical tests, including expertise in the expression of catalase, citrate, urease, nitrate reduction factors, coagulase, and other substances that qualitatively describe the metabolism of Pseudomonas aeruginosa. In addition, genomic analysis of the microorganism’s nucleic acids utilizing 16S rRNA sequence studies would have the highest reliability (Ferroni, et al., 2002). Ultimately, this extension may provide a more extensive view of an unknown specimen, but using the complete variety of tests is not an optimal identification strategy in the context of saving finances and time.
References
CDC. (2019). Pseudomonas aeruginosa in healthcare settings. CDC 24/7. Web.
Das, S. U. R. A. J. I. T., Lyla, P. S., & Ajmal, S. (2007). A simple scheme for the identification of marine heterotrophic bacteria. Thalassas, 23(2), 17-21.
Ferroni, A., Sermet-Gaudelus, I., Abachin, E., Quesne, G., Lenoir, G., Berche, P., & Gaillard, J. L. (2002). Use of 16S rRNA gene sequencing for identification of nonfermenting gram-negative bacilli recovered from patients attending a single cystic fibrosis center. Journal of Clinical Microbiology, 40(10), 3793-3797.
Pseudomonas aeruginosa micrograph. (2015). Microbiology Pictures. Web.