The identification if various forms of bacteria is an important part of clinical practice. To identify bacteria, the present phenotypic characteristics are usually compared with those of known bacteria. In the process of identifying bacteria a researcher needs to appreciate the fact that the characteristics may vary significantly. Furthermore some species within the “same genus may be seen to vary, for instance, capnocytophaga canimorsus is oxidase positive, while capnocytophsga ochracea is oxidase negative” (Pagana, 1998, p. 30).
For complete identification of bacteria, all the usual characteristics should be considered. Primary bacterial identification is normally done using simple tests such as morphology (usually shown by Gram stain), growth in the presence or absence of air, growth on various types of culture media, catalase and oxidase tests. Such tests are critical for the categorization of the bacteria into different groups that are of medical importance.
Recognition of the cultural characteristics is usually one of the best preliminary tests done to identify or categorize bacteria. Normally, when a single species is grown on specific media under controlled conditions it can be described using it’s characterize size, shape, consistency and sometimes pigment (Perera, 2006). Uniformity is typically seen when a single species of bacteria is cultured under controlled conditions. For instance, streptococco are small, usually 1mm in diameter, while those of staphylococci and enterobacteriaceae are larger as well as those of bacillus species (Health Protection Agency, 2008).
Different forms of bacteria often require varying conditions for growth to take place for instance strict aerobes only grow in the presence of oxygen while strict anaerobes grow in the absence of oxygen (Martinko, 2006). Facultative bacteria are capable of growing in both aerobic and anaerobic conditions. Bacteria may also be distinguished using the temperatures requirements for growth (Pagana, 1998). For instance, psychrophilic organisms grow at low temperatures with optimum growth being observed at temperatures between 10 and 30°C (Pagana, 1998). Mesophilic organisms grow at temperatures at temperatures between 10 and 45°C with optimum growth being observed at temperatures between 30 and 40°C (Pagana, 1998).
This study seeks to make use of different types of culture media and biochemical tests to identify a mixture of two unknown bacteria.
Materials and method
Materials
Phenol Red glucose fermentation broth with Durham tube
Phenol Red lactose fermentation broth with Durham tube
Phenol Red mannitol fermentation broth with Durham tube
Phenol Red sucrose fermentation broth with Durham tube
Lysine decarboxylase broth with Brom cresol purple
Arginine decarboxylase broth with Brom cresol purple
Ornithine decarboxylase broth with Brom cresol purple
Klieger’s Iron agar
Simmon’s citrate agar slants with Brom thymol blue
Urea broth
Tryptone broth for Indole test
Glucose peptone broth for Methyl red test
Glucose peptone broth for Voges-Proskauer (VP) test
Motility test medium with semisolid agar
Gelatin medium
Oxidase reagent
Catalase (hydrogen peroxide) reagent
Nitrate broth
OF (oxidative/fermentative) deep medium with Brom thymol blue
Phenylalanine deaminase agar slants (using the FeCl3 reagent)
Coagulase plasma
Bile esculin slants
Skim milk agar plates
Starch agar plates
Sodium chloride (6.5%) tolerance broth with Brom cresol purple
Sodium hippurate broth
DNase agar
Levine Eosin methylene blue agar
Mac Conkey’s agar plates
Mannitol salts agar plates
Staphylococcus medium 110 plates
Staphylococcus medium 110 broth
Hektoen enteric agar plates
Blood agar plates
Possible organisms ( Coliform and non coliform bacteria)
K. pneumoniae Salmonella typhimurium
E. coli Shigella. flexneri
E. aerogenes Pseudomonas. aeruginosa
Bacillus cereus
Streptococcus pyogenes
Alkaligenes faecalis
Proteus vulgaris
Serratia marcescens
Micrococcus luteus
Staphylococcus aureus
Bacillus subtilis
Methods
- A mixture of two isolates of bacteria were inoculated using a 4 phase streak onto 1 Mac Conkey’s Agar plate, 1 EMB agar plate, 1 hektoen enteric agar plate, 2 tryptic soy agar plates and 2 brain heart infusion agar plates.
- One of the tryptic soy agar plates and one of the brain heart infusion agar plates was incubated at 30°C, while the rest were incubated at 37°C.
- The plates were examined for growth after an incubation period of 1to 2 days.
- The morphological characteristics exhibited by the different plates were examined in order to try and identify the unknown bacteria.
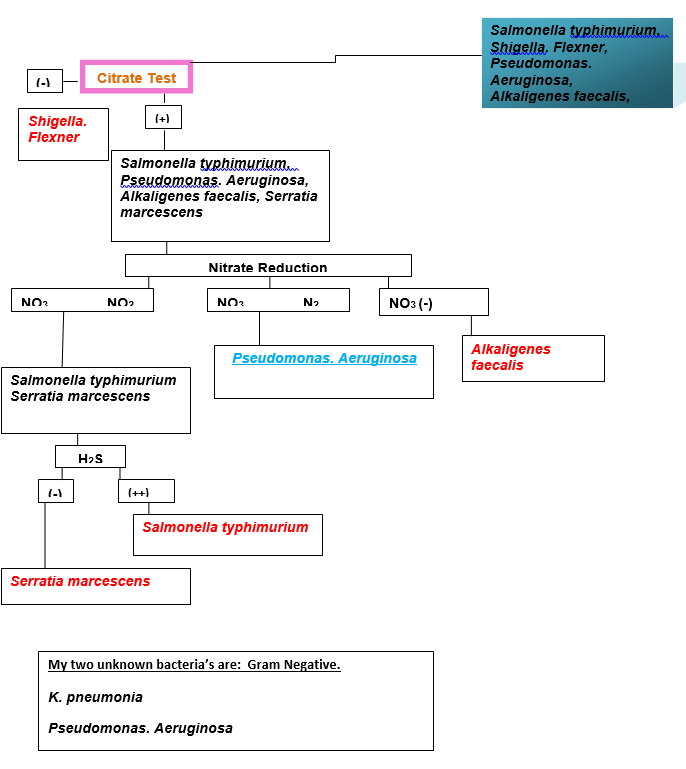
- A dichotomous key was constructed and biochemical tests carried out to identify the two unknown bacteria.
Results
Table 1: Colonial morphology.
Discussion
K. pneumoniae is a widely recognized opportunistic coliform pathogen and as an agent of pneumonia (Pagana, 1998). This is “a Gram-negative, non-motile, encapsulated, lactose fermenting, facultative anaerobic, rod shaped bacterium that is found in the normal flora of the mouth, skin, and intestines” (Martinko, 2006, p. 102). K. pneumoniae is can be identified using biochemical investigation. The bacterium grows on different types of media, on Mac Conkey agar it produces pink colonies which are a characteristic of many lactose fermenting bacteria. When indole test is performed it shows negative results and thus further investigation is required (Pagana, 1998).
When Urease test is conducted, the results are positive but again this is a characteristic of another bacterium referred to as Serratia marcescens (Pagana, 1998). Motility testing is the final test required to investigate whether the bacteria is K. pneumoniae which is non motile while Serratia marcescens is motile. In addition “K. pneumoniae is ecancapsulated, appears as mucoid colonies that tend to string when lifted with a wire loop” (Perera, 2006, p. 5).
Pseudonomas aeruginosa is a common bacterium that is capable of causing disease in animals (Martinko, 2006). The bacterium is a gram negative rod that appears colorless on Mac Conkey medium (non lactose fermenters) (Martinko, 2006). It can be differentiated from other gram negative bacteria that show colorless colonies on Mac Conkey agar using biochemical tests or examination of pure colonies using a fluorescent lamp (Health Protection Agency, 2008).
Using the biochemical tests one begins with the indole test of which positive results will indicate the presence of proteus vulgaris. Negative results may indicate the presence salmonella typhirium, alcaligenes faecalis or pseudonomonus aeruginosa and thus further investigation is required (Perera, 2006). Methyl red testing is done to rule out salmonella typhirium and therefore only two possible bacteria remain (alcaligenes faecalis or pseudonomonus aeruginosa) (Health Protection Agency, 2008). The reduction of nitrates to nitrites is almost a confirmation test for P. aeruginosa (Perera, 2006). However other tests can be performed such as growth at 42°C and the observation of motility (Pagana, 1998).
There are different types of media that are used in the cultivation of bacteria. For the purpose of this paper the following media will be described: Eosin methylene blue (EMB) agar is a selective agar that contains the dyes eosin and methylene (Perera, 2006). The aniline dyes in the agar inhibit the growth of gram- positive organisms (Pagana, 1998). The media can also be use as a differentiating media as the lactose in the media is metabolized by lactose fermenters to release acid by products (Pagana, 1998). Organism that produce strong acid such as Escherichia coli result into the production of a metallic green sheen (Perera, 2006).
While those that result into poor fermentation of lactose produce pinkish purple colonies (Perera, 2006). The EMB agar plate used in this practical had a mixture of pinkish and dark colonies which are a characteristic of the two unknown bacteria pseudomonas aeruginosa (non-lactose fermenters) and klebsiella pneumoniae (lactose fermenting) respectively (Pagana, 1998).
Mac Conkey agar “contains lactose, bile salts, neutral red and crystal violet” (Pagana, 1998, p. 84). Mac Conkey agar can regarded as a selective media due to the fact that the crystal violet and bile salts contained in it inhibit the growth of Gram –positive bacteria. Acid production by lactose fermenters bacteria results in the lowering of the pH below 6.8 (Perera, 2006). This makes the neutral red to turn from colorless to red color, therefore “Mac Conkey is a deferential media on which lactose fermenting colonies appear red (or pink) while the no lactose fermenters are colorless” (Perera, 2006, p. 45).
The Mac Conkey plate used in the experiment had both pink and colorless colonies characteristic of klebsiella pneumoniae (lactose fermenting) and pseudomonas aeruginosa (non-lactose fermenters). Pseudomonas aeruginosa can be detected rapidly using ultraviolet light that makes the colonies to fluoresce but this was not utilized in the above practical. (Pagana, 1998)
Tryptic Soy Agar “(TSA, Casein Soya Bean Digest Agar) is a complex medium that is used for cultivation and isolation of fastidious bacteria, yeast and molds” (Martinko, 2006, p. 69). Tryptic Soy Agar plates that are intended for bacterial detection are incubated at 30 to 37°C degrees for up to 3 days (Health Protection Agency, 2008). Several bacterial species including pseudomonas aeruginosa and klebsiella pneumoniae will produce medium sized, slightly yellowish colonies when incubated on a Tryptic Soy Agar plate (Pagana, 1998). In the experiment carried out, slightly yellowish colonies with sizes varying from medium to large were observed in the TSA plate that was incubated at 37°C (Perera, 2006).
Hektoen Enteric (HE) agar plate is a “moderately selective medium that is used in qualitative procedures for the isolation and cultivation of gram-negative enteric microorganisms, particularly shigella from clinical and nonclinical specimens” (Pagana, 1998, p. 108). The media inhibits most lactose fermenting bacteria is but they sometimes grow to form yellow colonies. Pseudomonas aeruginosa is not inhibited and thus grows to from small flat colonies which appear green to brown in color.
Brain heart infusion agar is an enriched non-selective medium that is often used for the cultivation of various fastidious organisms including yeast and molds (Perera, 2006). The media is mainly used in bacteriology for its ability to cultivate many anaerobic species. Brain heart infusion agar can be used in the cultivation of Pseudomonas aeruginosa which is capable of growing in hypoxic conditions (Perera, 2006).
Conclusion
This study sought to establish two mixed unknown bacteria. The morphological characteristics were identified after incubation in the following types of media “Brain heart infusion agar, Hektoen Enteric (HE) agar, Tryptic Soy Agar, Mac Conkey agar, and Eosin methylene blue (EMB)” (Pagana, 1998). A dichotomous key was constructed, biochemical tests carried out and the two unknown bacteria identified as pseudonomonus aeruginosa and klebsiella pneumonia (Perera, 2006).
References
Health Protection Agency. (2008). Introduction to the preliminary identification of Medically important Bacteria. London : National Standard.
Martinko, M. (2006). Brock Biology of Microorganisms. New York: Prentice Hall.
Pagana, T. (1998). Manual of Diagnostic and Laboratory Tests. Missouri: Mosby.
Perera, N. (2006). Identification of Bacteria. Leicester : University hospital of leicester.