Introduction
What is the atmosphere? “Envelope of gases that surrounds the Earth” (Origin of the Earth’s atmosphere, n.d.). Another definition is as follows: “The Earth’s atmosphere (or air) is a layer of gases surrounding the planet Earth that is retained by the Earth’s gravity” (Priyadarshi, 2009). It is a dense composition of chemical compounds that act as a reservoir for sustaining life forms. Most of the atmosphere about 97% is found 30km away from the earth’s surface with the rest fading into space seeing, as it has no outer boundary (Origin of the Earth’s atmosphere, n.d.). The main purpose of the atmosphere and all its strata is to protect the earth’s living organisms by absorbing ultraviolet (UV) solar radiation or rays through the Ozone stratum. These layers are also responsible for warming the earth’s surface through heat retention (greenhouse effect) and regulating night and day temperatures on the earth.
The earth’s atmosphere was quite inhospitable and uninhabitable before it evolved into what it is today. The formation of the atmosphere was probably due to the outgassing of the atmospheric components from the mantle through a volcanic eruption. It will look at the steps involved and how the atmosphere came to be what it is today. In addition, the concept of the Hadean-Archaean environment will be looked at in relation to the evolution of the atmosphere. The evidence of the evolution of the atmosphere is also espoused. The components of the atmosphere, which are the various gases, will be shown and how they contribute to the environment. Lastly, the structure of the atmosphere will be explored.
The earth’s early environment could not support the life of most species of plants and animals. This was mainly because of the following factors:
- Primordial heat in the planet itself.
- Factors such as the decay of short-lived radioactive elements create an exothermic reaction.
- The collision of particles in the formation of planets through accretion
- The composition of the atmosphere, which was primarily hydrogen and helium.
The cooling of the earth’s atmosphere due to dispersal or dissolution of primordial heat, volcanic outgassing- which leads to accumulation of new atmospheres (second atmosphere) and condensation and accumulation of surface water. These must have been a source of very cool temperatures that were not conducive for life as they fell to subzero temperatures.
The consequences of the heat were the instability in the earth’s crust and the surface temperature being too high to sustain liquid water in that form as well as constant eruptions.
The First Theory on Evolution of the Atmosphere
The earth’s atmosphere originally constituted elements such as carbon dioxide, nitrogen, methane, ammonia, hydrogen, and water vapor. This atmosphere was mainly devoid of oxygen (ergo could not support life) and it is commonly referred to as the reducing atmosphere.
First Atmosphere
This atmosphere was comprised of mostly hydrogen and helium although other light gases such as neon, argon, and xenon, which have become rare in the modern atmosphere (comprising about 1% of the atmosphere). This is because of the dissipation of the elements into the atmosphere because of factors such as the earth’s gravitational pull not being strong enough to keep the lighter gases and the effect of the Van Allen Belt (magnetosphere). This is the earth’s magnetic field, which operates in a way that deflects solar wind (The Earth’s atmosphere, n.d.).
Another reason why this primitive atmosphere evolved into the second was binding with mineral elements or these gases escaping the earth’s atmosphere into the universe. The subsequent differentiation of the earth’s core led to the retaining of heavier gases like CO2. These gases probably existed in the cosmic cloud (or solar nebula) that marked the formation of the planetary system, as we know it.
Second Atmosphere
This second evolutionary level was formed by volcanic outgassing. This atmosphere was probably comprised of these chemical elements: H2O, CO2, SO2, CO, S2, Cl2, N2, H2, NH3 (ammonia), and CH4 (methane). Oxygen was still lacking in this stage of evolution, as O2 is not a component of volcanic gases.
Addition of O2 to the atmosphere
This is the last stage of evolution in which free oxygen is elevated to levels that support living organisms. The current percentage of free oxygen in the atmosphere is
~21%. These levels could have been reached by the occurrence of these processes:
- Photosynthesis by cyanobacteria (or as they are commonly referred to, blue-green algae) and later higher plants. The combination of CO2 + H2O + sunlight produces organic compounds + O2.
Photochemical dissociation whereby the water molecules in the atmosphere and in the terrestrial domain(ocean) are broken down by UV rays or ultraviolet rays from the sun and converted into oxygen and free ions of hydrogen. This is probably responsible for 1-2% of the current levels of free oxygen. This is the amount necessary to make up the OZONE (O3), which protects from UV rays.
Photolysis of water molecules – This process forms hydrogen peroxide, which combines with other elements to form a redox reaction, which perpetuates life in the recurrence of the production of free oxygen.
There was very little free oxygen (<1% of current levels). It was known as the Archean Earth or the “Age of the prokaryotes” (Archean Life, n.d.). In the earliest forms of life, cyanobacteria such as stromatolites produce an essential waste product called oxygen that was mainly consumed in chemical weathering through oxidation, leaving the residual oxygen as free oxygen. Evidence of this effect is the existence of red beds (continental siliciclastic deposits) which are highly oxidized minerals formed from the mineral hematite.
During the Proterozoic period (2.3 Ga), the cyanobacteria had multiplied in large numbers thus elevating the oxygen levels to about 10%. By 400 Ma the levels had risen to the current approximate value of 21% (Origin of the Earth’s atmosphere, n.d.).
It is important to note in the history of the atmosphere that, the atmosphere contributed to the formation of the oceans(hydrosphere). This was through the accumulation of water molecules in the atmosphere, which in turn led to high precipitation levels.
The Second Geological Theory Hadean Archaen Environment
According to the Hadean-Archean concept of geology (, rock records show that the earth’s atmosphere was formed in a sequence of the following stages:
The Primary atmosphere was thought to be composed of mainly nitrogen and trace elements of carbon dioxide, carbon monoxide, and methane. This part or stage of the atmosphere consisting of these elements may have been blown off the earth’s atmosphere through the moon forming an impact approx. 100 m.y (million years) after the consolidation of the earth’s crust (Holland 1984, 1994).
This subsequent layer was comprised of mainly CO2 and other chemical components such as CO, NH3, N2, and probably a limited amount of CH4. The last part of the evolution was the addition of oxygen into the environment through the oxidation processes of rocks on the earth’s surface, especially ferrous metals. This explains why most of the oxygen is locked into the earth’s core in the earth’s crust. Another reason why the current atmosphere has a much higher percentage of free oxygen is the processes of photolysis of water molecules into hydrogen peroxide and d oxygen as mentioned earlier. The modern-day atmosphere is therefore known as the oxidizing atmosphere.
The Third theory on the Evolution of the Atmosphere
This is the most recent theory that postulates that the current levels of oxygen in the atmosphere are due to the introduction of oxygen through comets and other planetesimals like asteroids, meteors, and meteorites which contain a substantial amount of volatile materials.
“A shift in Jupiter’s orbit around 4.5 billion years ago may have jarred the Kuiper Belt, flinging icy comets at the Earth” (Barley, 2009). “Ancient Earth was strewn with huge volcanoes spewing out gas, but our research shows that the real source of Earth’s first atmosphere was actually outer space,” says Chris Ballentine, a Harvard-Smithsonian Center for Astrophysics in Cambridge, Massachusetts (Barley, 2009).
“Earth’s atmosphere came from outer space, its gases arriving in a meteorite bombardment billions of years ago, scientists claimed yesterday,” said a scientist Holland at the Harvard-Smithsonian Center for Astrophysics in Cambridge, Massachusetts who is also a college of Ballentine (Smith, 2009).
These are the scientific research findings that have led the world to rethink the evolution of the earth and consider the effect of foreign objects entering the earth and contributing to the addition of gaseous components into the atmosphere.
Evidence of the Evolution of the Atmosphere
The evidence of the evolution of the earth’s atmosphere may be in form of biological evidence or rock record in geology.
Biological evidence
In the present day, most life forms (humans and plants alike) use oxygen to carry out life processes such as respiration, photosynthesis, and other metabolic processes. Seeing as the percentages were in an amount of less than one percent, the chemical processes could have been inhibited. For example, in humans, the processes that yield amino acids require a certain amount of oxygen. The process of breathing most importantly would be impossible.
Another example that attests to this is the fact that oxygen inhibits the growth of a majority of primitive living bacteria like photosynthetic bacteria like algae and lichens, bacteria that produce methane as a waste product of their metabolism processes, and others that get their working energy from fermentation processes. This shows that the primordial forms of life were most probably such types of bacteria. This also explains why most of such bacteria today live in anoxic (low oxygen content) environments like swamps among others.
Evidence from Rock Records
As discussed earlier, iron has a high affinity for oxygen. In studying rock records of ferrous minerals (found in ocean beds as deposits) such as magnetite or the iron ores like hematite, one can infer that there was indeed an oxidation process that binds up oxygen and limits the amount of the present free oxygen. These iron ore formations are called BIF or Banded Iron Formation.
Secondly, the existence of minerals found in non-oxidizing environments such as Fool’s Gold or Pyrite, or Uranitite (UO2) proves that there was once a reducing atmosphere devoid of free oxygen. These minerals easily dissolve out of the environment in this current oxidizing atmosphere.
Lastly, the existence of red beds, which as mentioned before are deep, are rooted in the crust of the earth. This core is mostly composed of hematite explaining why it is red in color.
The Chemical Composition of the Atmosphere
The following is a diagrammatical representation of the current chemical compositions of the earth’s atmosphere.
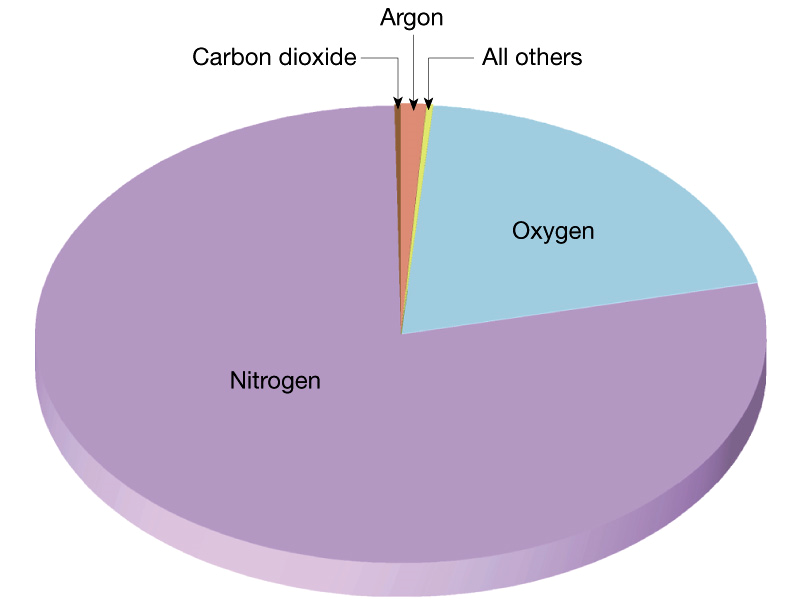
The chemical composition of the atmosphere today is as follows:
The highest composition is Nitrogen or N2 followed by Oxygen (O2), which makes up about a fifth of the total at 21%, then Carbon Dioxide (CO2) with a percentage of 0.003% with other miscellaneous inert gases like krypton, argon, hydrogen, water vapor and helium at the remaining less than one percent.
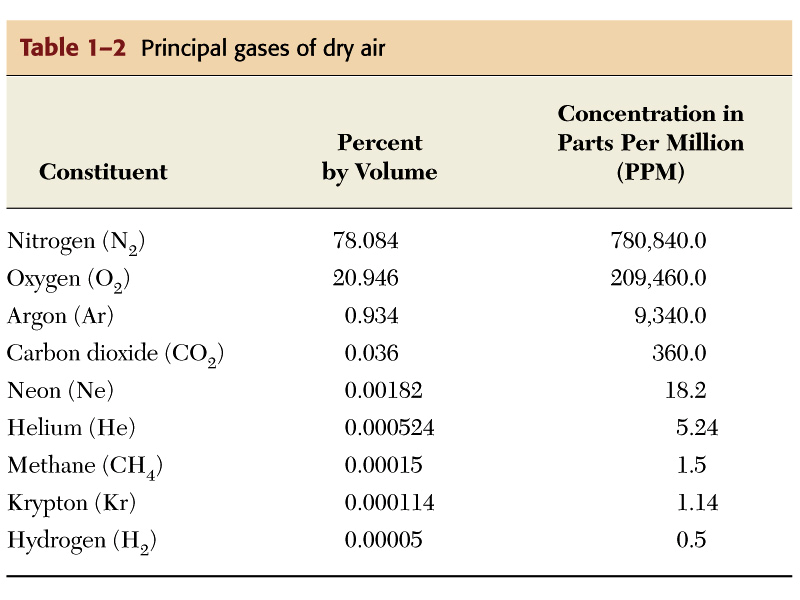
The table above gives the exact percentages of the chemical composition of the atmosphere in terms of the gasses present. The percentage of water vapor is not given however, but it accounts for the remaining one percent of miscellaneous gases.
The structure of the Current Atmosphere
The following is a representation of the vertical structure of the layers of the atmosphere from the lower terrestrial level into space.
The Troposphere
This is the immediate level, which is closest to the earth. It is where most of the weather takes place. It rises to about ten kilometers above the earth’s surface and the atmospheric pressure o top of the troposphere is approximately ten percent of that of sea level at 0.1 atmospheres. It is separated from the next level by the tropopause, which acts as a buffer zone (The Earth’s Atmosphere, n.d.)
The Stratosphere and the Ozone
This is the next level that follows the troposphere. Its inception is from about 10km above the earth’s surface to altitudinal height peaking at about 50km above the earth’s surface. The airflow here is mostly horizontal. The upper part of the stratosphere contains the Ozone layer which s we said before is important for protecting the earth from harmful UV radiation and to regulate and stabilize temperatures during the night and day time. It is composed of the Ozone, which is a highly reactive form of oxygen represented by the chemical formula, O3. The depletion of the Ozone layer is a delicate environmental concern issue. Man-made fluorocarbons and other pollutant activities have been the key sources of this depletion. The Ozone layer may continue to deteriorate to dangerous levels if production of oxygen through such processes as photosynthesis is reduced (The Earth’s Atmosphere, n.d.).
The Mesosphere
This is the subsequent level after the stratosphere. It is also separated by the buffer zone commonly known as the stratopause. It begins from 51 km to about 85 km above the earth’s surface. The top of the mesosphere is the coldest part of the Earth’s atmosphere averaging temperatures of as low as -90 degrees Celsius or -130 degrees Fahrenheit. It is difficult to measure information about the mesosphere seeing as it is too high but from the use of sounding rockets, these facts have been uncovered (Russell, 2008):
- Meteors and meteorites burn up in this part of the atmosphere.
- The existence of special kinds of clouds called nocticulent clouds that form at this elevated level of high altitudes in the atmosphere close to the North and the South Poles.
- The occurrence of special types of lightning called ‘sprites’ or ‘elves’ that are seen in the mesosphere above the thunderclouds of commonly found in the troposphere.
- The air in the mesosphere is so thin that atoms and molecules of various gases rarely fall upon or happen upon each other and combine. There are some that separate (for example, those with nitrogen and oxygen in them).
- The movement of air in the mesosphere is due to waves (similar to that of the ocean) that occur in the air in the lower atmospheric strata; that is the troposphere and stratosphere. These waves also contain energy, which is pushed or carried into the mesosphere.
The Ionosphere or Thermosphere
This is the summit in the atmospheric strata and final part of the atmosphere. It is where many atoms have become ionized such that they have an electrical charge. It is where the phenomenon of aurora (or the Northern Lights) takes place and where most of the Sun’s energy abundant photons are absorbed into the atmosphere. The ionosphere is also accountable for the reflection of radio waves making long- distance communication via radio an achievable task. The ionosphere can be measured by the density of charged chemical particles called electron. This tells us about the degree of ionization that has occurred in this layer of the atmosphere. This electron density is the result of the solar wind caused by charged particle wind from the Sun. The ionosphere is found at 85 to 90 kms above the Earth’s surface and continues on into space (The Earth’s Atmosphere, n.d.).
The following is a diagrammatical representation of the earth’s atmosphere.
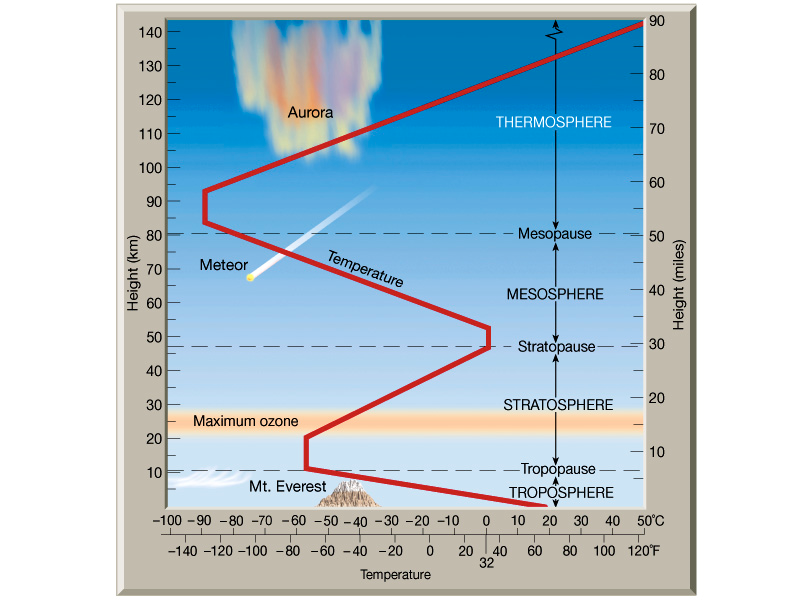
With increasing altitude there is a marked decrease in temperature as represented in the diagram above. This phenomenon explains why the mesosphere has been found to be the coldest layer of the earth’s atmosphere. The reason why this is not true for the ionosphere is probably due to the solar wind and electrical activity of the photons from the Sun.
Conclusion
In conclusion, the earth’s atmosphere has come a long way since the anaerobic primordial atmosphere of the cyanobacteria. Their contribution to adding free oxygen to the atmosphere has been a great factor in shaping the atmosphere, as we know it. The production of oxygen as a waste product is the prime reason why the Ozone was made and the subsequent twenty one percent of free oxygen, which sustains the life of most life forms. The subsequent oxidation of minerals in the earth’s crust is the reason why there is the current levels since these minerals left the residual oxygen as free oxygen.
The theories involved in explaining the evolution have also progressed over time. Recent studies conducted by scientists prove the contribution of comets and other planetesimals. These bodies provide volatile gases such as helium, hydrogen and other inert gases that formed the first atmosphere. This was later dissipated into space giving rise to the second atmosphere.
The main evidence of the evolution of reducing atmosphere to the oxidizing atmosphere is of a biological nature and of geological nature. It is discussed above that life cannot live without oxygen and therefore the current atmosphere must evolved from the anoxic environment of bacteria into the current oxygen rich state conducive for the sustaining of living organisms. In addition, red beds have shown that oxidation occurred to produce this environment. This is discussed in relation to the Hadean-Archaen environment.
The composition of the current atmosphere is detailed in terms of percentages.
The structure of the oxidizing atmosphere as we know it is also examined giving the key characteristics of each of these layers mentioned above: troposphere, stratosphere, mesosphere and the thermosphere. Their contribution to the geospheres is examined. For example, the stratosphere contains the Ozone layer, which protects the earth from UV radiation.
Lastly, it is crucial to point out that this atmosphere is changing everyday due to the effects of pollution. The Ozone would be depleted completely if processes such as photosynthesis came to a halt. However, it is important that people become more environmentally sensitive to the environment to ensure the longevity and maintenance of our atmosphere, as we know it.
Reference
Barley, S. (2009). Our atmosphere came from Outer space. New Scientist. Web.
Fishbaugh, K. E., Lognonné, P., Raulin, F., Des Marais, D. J. & Korablev, O. (2007).
Geology and Habitability of Terrestrial Planets. Space Science Series of ISSI. Springer. 2010. Web.
Origin of the Earth’s Atmosphere. (n.d.). The Atmosphere Origin and Structure. 2010. Web.
Priyadashi, N. (2009). The Evolution of the Earth’s Early Atmosphere. Web.
Russell, R. (2008). The Mesosphere. University Corporation for Academic Research. Web.
Smith, M. (2009). Earth’s Atmosphere ‘created by meteorites’. Scotsman.com. Web.
Tarbuck, E.J. & Lutgens, F.K. (1989). The Atmosphere. Prentice Hall, New Jersey. Web.
The Earth’s Atmosphere. (n.d.). The Earth’s Atmosphere. 2010. Web.