Introduction
Pancreatic cancer has been known to result from active smoking, but the role passive smoking plays in the development of pancreatic carcinogenesis is not clear. Cigarette smoking has been identified by many studies as the main risk factor for this disease. According to Bao et al. (2009), smokers have a higher likelihood of developing pancreatic cancer. In most cases, women make up the highest number of passive smokers, although 15% actively smoke while pregnant. Moreover, environmental tobacco smoke (ETS), passive smoking, has an effect similar to active smoking because it contains some carcinogenic components in mainstream tobacco smoke and can increase the risk of pancreatic cancer.
So far, there is inconsistent and limited epidemiologic evidence concerning the connection between pancreatic carcinogenesis and passive smoking. One out of 12 studies done on this relationship has a future design, and most of them have had relatively fewer cases, mostly below 12. Therefore, the study by Bao et al. (2009) used a larger cohort of U.S. women to examine how the risk of pancreatic cancer is linked to passive smoking. Their focus was on more detailed information regarding the passive smoking history and other risk factors of the disease, and this is the only study done particularly for maternal smoking.
Bao et al. Research Review
The research had a prospective design, and its population comprised 121,700 U.S. women aged between 30 and 52 years. The researchers took their detailed information, including a history of active smoking, and using questionnaires, in 1976 and twice-yearly until 2006, and the overall follow-up rate was 90% (Bao et al., 2009). Women a cancer history up to 1982 and those that did not return their questionnaires were excluded from the study, and only 86,673 participants were eligible. The Human Research Committee at Brigham and Women’s Hospital approved this study (Bao et al., 2009).
The exposure definition entailed the medical conditions reported on the two-yearly follow-up questionnaire. The researchers obtained permission to access the pathology reports or related medical records of the women that reported pancreatic cancer diagnosis. They identified 384 pancreatic cancer cases between the return of the 1982 questionnaires and the end of 2006, and medical records confirmed 86% of them. Secondary sources, such as telephone interviews of family members, physicians, or death certificates, confirmed the remaining proportion of cases. However, in the analysis, both types of cases were included, and they yielded similar results.
Exposure assessment involved the evaluation of environmental tobacco smoke. This included the participants’ reports of their exposure to passive smoke in their childhood and when the study was being carried out, and this information was obtained using the 1982 questionnaire (Bao et al., 2009). The specific question was whether their parents were smoking when they lived with them or if their work or home, they were exposed to secondhand smoke, and for how long.
The outcome description in this research entailed the researchers using the regression of Cox proportional hazards stratified by calendar time and age to estimate the confidence intervals (CI) and relative risks (RR). This approach enabled them to create a metric of time concurrently accounting for these stratifications. The outcome assessment was based on previous exposure to secondhand smoke. The comparison group comprised those participants whose parents were nonsmokers. The researchers then calculated the RRs of the women whose parents smoked then made comparisons of the risk between the participants exposed to smoke at that time at the workplace or at home and those not exposed.
Results
Based on the statistical tests performed, the researchers found a positive connection between early exposure to ETS and pancreatic carcinogenesis (Bao et al., 2009). In their statistical tests, the researchers adjusted for active cigarette smoking, diabetes, and BMI in multivariate models since these are the possible or established risk factors for pancreatic cancer. The analysis was restricted to never-active smokers, totaling 37,757, and the measure of association calculated was paternal and maternal smoking (Bao et al., 2009). The following figure shows a summary of the results of adjusted RR.
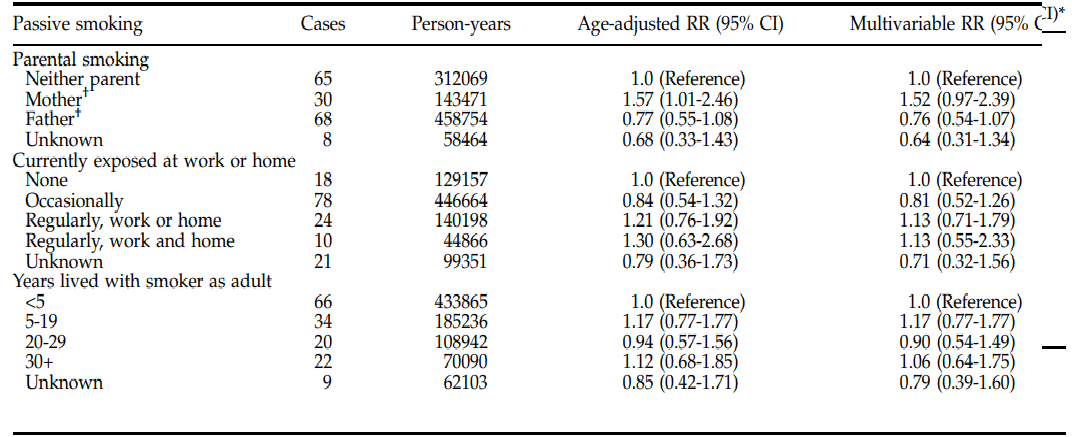
From this epidemiologic evidence, several mechanisms help explain the association between pancreatic cancer and maternal smoking. For example, the carcinogens in ETS result in adverse genetic effects, stimulating proliferation and mutation of the cells and genes, respectively. Moreover, ETS induces a chronic inflammatory response; thus, the smoke triggers pancreatic carcinogenesis. Additionally, passive smoking has a relatively higher intensity than paternal smoking because children spend more time with their mothers. Many similar studies had been done on this topic, but there was a null association between pancreatic cancer and passive exposure during childhood. Some of the reasons for this include asking vague questions resulting in exposure misclassification. The only limitation of this study was the inability to examine the difference in association with passive smoking depending on gender because all participants were women.
Conclusions and Recommendations
In summary, there is a connection between pancreatic carcinogenesis and the role of early exposure to environmental tobacco smoke. Moreover, there is a positive relationship between pancreatic cancer risk and maternal smoking. The results from related studies were not consistent with this research as others had a null association between the exposure and the disease, although their approaches were different. Future research should focus on the relationship between utero exposure and maternal smoking. The study population needs to have shared characteristics, such as exposure to paternal or maternal smoking in childhood, and all studies must not adjust for a certain confounder. Lastly, exposure should be defined as direct contact with environmental tobacco smoke resulting from passive smoking.
Villeneuve et al. Research Background
Many previous studies have concluded that the major risk factor for pancreatic cancer is cigarette smoking. Still, none of them has attempted to examine how this malignancy is related to ETS. Villeneuve et al. (2004) conducted a population-based case-control study to examine how exposure to ETS is related to pancreatic cancer. In Canada, the fourth leading cause of death is pancreatic cancer, and less than 5% of those diagnosed with this malignancy survive five years (Villeneuve et al., 2004). Numerous carcinogenic constituents are contained in passive and active tobacco smoke components, increasing the risk of cancer. For example, there are 43 known carcinogens in environmental tobacco smoke (Villeneuve et al., 2004). The particulate sizes of environmental tobacco smoke are smaller because most of its constituents exist in vapor form, and their uptake differs between passive and active smokers. This study aimed to explore how the incidence of pancreatic cancer is related to the active intake of environmental tobacco smoke.
Villeneuve et al. Research Review
The study design of this research was a population-based case-control. The study population included 20,730 Canadians who had been diagnosed with one type of cancer from the 5,039 controls (Villeneuve et al., 2004). The National Enhanced Cancer Surveillance System (NECSS) collected data in 8 provinces under study in Canada between 1994 and 1995. By 1997, all the data had been collected in all the eight provinces under study (Villeneuve et al., 2004). The participants included in the study were those who had completed the questionnaire in the period of data collection and were at least 30 years old. Conversely, the excluded ones were the military personnel and their families, as non-public plans cover them.
The methods previously used by the NECSS were applied in the evaluation of the connection between other types of cancers and environmental tobacco smoke.
Exposure assessment included questions relating to the participants’ history of passive smoking exposure. For example, the data required was the number of regular smokers who usually lived within the home and those in their immediate work environment. The researchers restricted their analysis to those exposed to ETS for at least 75% of their life (Villeneuve et al., 2004). The aim was to reduce the likelihood of misclassification of environmental tobacco smoke exposure.
Results
The researchers performed two analyses to determine how pancreatic cancer is associated with ETS. The first one was a model relationship for novel smokers, based on the nature and period of the exposure before conducting risk analyses for females, males, and combined sexes. The second analysis was an examination of whether excluding the category of never active smoker made the risks more pronounced. The 95% confidence intervals and odds ratios were computed using logistic regression (Villeneuve et al., 2004). The results indicate that there were few cases of pancreatic cancer, 16, among the never smokers, but there was a relatively higher association between the malignancy and exposure to ETS. There were adjusted multivariate models for the effects of pancreatic cancer’s other risks. The measures of association calculated were lifetime passive exposure, and there was no significant relationship between the occurrence of pancreatic cancer and the indices of ETS. The figure below shows the adjusted results.
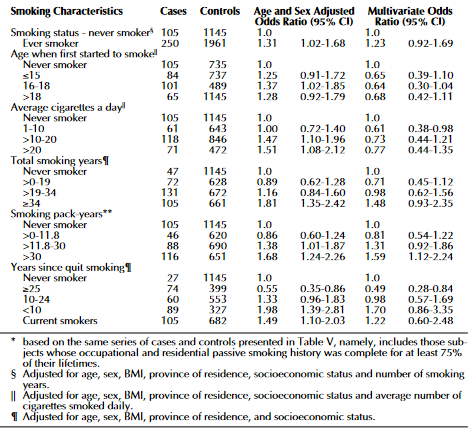
Overall, the case status and the extent of exposure to ETS showed a statistically significant relationship. It was more accurate to use the exposure period to determine the risk of pancreatic cancer than using the amount of smoke. It was not possible to capture the variations in the individual ETS exposure levels because the passive exposure measures were only basic, which was the first limitation of the study. The second one was that their research, being case-controlled, might have been affected by recalling bias because the profile data collection for the case population is different from people who had not been diagnosed with any cancer.
Conclusions and Recommendations
In conclusion, as it can be seen from the findings of this study, the association between ETS and pancreatic cancer is weak. The pancreatic cancer risk associated is confounded by environmental tobacco smoke exposures related to other epidemiological studies’ active smoking exposures. However, this observation should not be misunderstood since many other studies have recognized the most significant risk factor of this malignancy to be ETS. All researches should adjust to the confounder of excluding the never smokers since pancreatic cancer is mainly caused by active smoking. Moreover, the study population needs to be limited to active smokers or nonsmokers who have been exposed to secondhand smokers for at least 80% of their lifetime. Thus, the definition of exposure should be formed along these lines. Lastly, among the study population, few cases of pancreatic cancer amongst nonsmokers were found. Therefore, further studies need to be done to examine this association and explore how other types of cancers are related to environmental tobacco smoke since, from the medical records of the participants, such hints were identified.
References
Bao, Y., Giovannucci, E., Fuchs, C. S., & Michaud, D. S. (2009). Passive smoking and pancreatic cancer in women: A prospective cohort study. Cancer Epidemiology Biomarkers & Prevention, 18(8), 2292-2296. Web.
Villeneuve, P. J., Johnson, K. C., Mao, Y., Hanley, A. J., & Canadian Cancer Registries Research Group (2004). Environmental tobacco smoke and the risk of pancreatic cancer: Findings from a Canadian population-based case-control study. Canadian Journal of Public Health, 95(1), 32–37. Web.