Introduction and overview
Human body produces an array of serine proteases involved in protein digestion, blood coagulation, and homeostasis. Recently, another important function of proteases: trypsin, thrombin, coagulation factors Xa and VIIa, cathepsins, etc. in signal transduction has been elucidated, which involves cell surface receptors, termed protease-activated receptors (PAR1-4) (Ossovskaya & Bnnnett 2004). The epithelial, endothelial, connective tissue, smooth muscle, and neuronal cells are the common sites for signal transduction. These G-protein activating receptors are five trans-membrane helix proteins with an extended N’-terminal region, susceptible to proteolytic cleavage. Several proteases may recognize a single receptor and diverse receptors may coordinate a function triggered by a single protease. Specific PAR motifs are recognized by specific protease(s) and conserved sites are cleaved leading to the generation of tetherend ligand which binds to another conserved extracellular binding domain in loop II of the receptor. This triggers G-protein mediated downstream signaling events. Synthetic peptides with conserved tetherend sequences can mimic and serve as receptor agonists in absence of protease. PAR1 and 2 commonly regulate cellular functions in the cardiovascular system, notably blood coagulation, vascular homeostasis, and immune and anti-inflammatory responses.
PAR2 mediated signal transduction
The downstream signaling pathways were investigated in specific call lines using agonists, called activating peptides (AP). According to Traynelis & Trejo (2007), PAR2 evokes two different signaling pathways (Fig. 1); one by activating the G signaling proteins Gαq, Gαi, and Gα12/13, and the other by β–arrestins, which interact with PAR2 to replace the G proteins. PAR2 undergo phosphorylation, a mechanism of desensitization of activated receptor so that the receptor quits the G proteins and joins arrestins. A complex of activated PAR2-arrestin-extracellular signal-related kinase (ERK1/2)-raf1-mitogen-activated protein kinase (MEK1/2) is then formed, which scaffolds the entire module in endocytic, and the MAP kinase signaling pathway is activated. Internal PAR2 module quickly dephosphorylates and returns back to the cytoplasm for another round of signaling events. In both the pathways, a variety of cellular responses is triggered; though at a transcriptional level which molecules are the effectors is largely unknown. PAR2 downstream signaling appears to be on gene expression of phospholipase C, protein kinase C, c-Jun N’ terminal kinase, p38 MPK, nuclear factor κB, etc. linked to chemotaxis and cytoskeleton rearrangement.
Recently, Wilson et al. (2009) reported one such effector, termed cut homeobox1 (CUX1) transcriptional factor, acts as a repressor or activator of a number of genes responsible for cell identity, growth cycle, intercellular communication, and motility. PAR2 seems to modify CUX1 protein by phosphorylation and acetylation, thereby increasing DNA affinity.
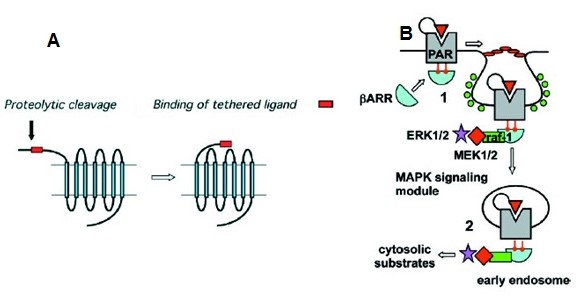
Internalization of PAR2 upon binding with β-arrestin and other proteins.
PAR2 in blood coagulation
PAR2 is activated by epithelial trypsin, mast cell tryptase, some leukocyte and cell surface proteases, and bacterial proteases. In the extrinsic pathway, the process is initiated from outside the blood vessels from tissue damage. Tissue factor (TF) or thromboplastin is produced in monocytes or keratinocytes from inflammation, which enters the vessels and interacts with endothelial factor activated VIIa. This complex binds to inactive X to convert to Xa. Factor Xa proceeds to activate factor V to Va, for the eventual transformation of prothrombin to thrombin. PAR2 recognizes weekly TF-VIIa and strongly TF-VIIa-Xa, and the latter being a protease, activates PAR2 in platelets to facilitate inflammatory coagulation.
PAR2 and inflammatory responses and immunity
The innate and adaptive immunity in response to infection relates to leukocytes (Shpacovitch et al. 2008). Immune response is derived by cells of circulatory system, e.g. neutrophils and/or the cells derived from leukocytes, viz. macrophages from monocytes. Neutrophils PAR2 activates Ca2+ intake and changes the shape to facilitate migration in extracellular matrix towards the inflamed/infected sites. L-selectin is a surface molecule preventing migration of neutrophils and PAR2 agonists prevent its synthesis. The other responses are release of interleukins-6, -8, -1β, lactoferrins and cytokines. Expression of Mac-1 and VLA-4 integrin genes responsible for cellular adhesion is up-regulated.
No receptor is found on eosinophil surface though expression of PAR2 takes place, which seems to be responsible for interleukin release, superoxide production and eosinophil degranulation. Mast cell tryptase and bacterial proteases activate PAR2 and as eosinophils phagocytize and react to allergens. Monocytes and monocyte-derive macrophages also express PAR2 and increase internal Ca2+ and release interleukins. The most notable action of PAR2 is in mast cells producing tryptase, which by activating the receptors produce histamines in response to tumor necrosis factor-α and other inflammatory agents. Inflammation and infection directly affects localized coagulation mediated through PAR2.
Role of PAR2 in vascular physiology
PAR2 is present in vascular endothelial and smooth muscle cells. In physiological state PAR has no role in blood flow. According to Cicala (2002) and Hirano (2007), inflammation and sepsis are primary factors for dynamic changes in PAR levels. PAR2 enhances endothelial vaso-relaxation by way of its increased synthesis and surface accumulation in endothelial cells. The receptor induces Ca2+ intake, stimulating Ca2+ stimulatory nitric oxide synthase. By an unknown mechanism PAR2 also participate in nitric oxide-independent vasorelaxation. Inflammatory mediators like tumor necrosis factor-α, lipopolysaccharides etc. increase endothelial PAR2 expression. Vasorelaxation in peripheral vasculature increases blood supply to affected sites.
Vascular damage also up-regulates PAR2 expression especially in arterial smooth muscles. Smooth muscles of coronary arteries and aorta express PAR2, but saphenous vein and myocardial smooth muscles do not. This suggests modulation of peripheral vasculature subject to injury and inflammation. Unlike endothelium, PAR2 activation stimulates smooth muscle contraction and proliferation, both coordinated by Ca2+ intake. Vascular damage also attracts mast cells which release tryptase to activate PAR2 to induce proliferative response, which is normally mediated through coagulation factor Xa.
The overall impact of PAR agonist is hypotension, affecting the peripheral rather than myocardial tissues. Cardiac hypertension is a concomitant reflex action. As PAR2 is expressed even in unstressed vascular tissue most likely it plays a role in vascular homeostasis and upon injury inflamed tissue encourages PAR2-mediated coagulation fibrinolysis.
Role of PAR in cardiovascular diseases
PAR’s participate in coagulation, inflammation and vascular homeostasis. Platelet aggregation leads to clot formation but perturbed aggregation cause atherosclerotic plaques and thrombus formation (Leger, Covic & Kuliopulos 2006). Entangled macrophages secrete cytokines and matrix metaloproteases. To overcome this pathological condition thrombin is introduced, which as agonist of PAR1, 3 and 4 markedly suppress the thrombus formation and act as anticoagulant.
Another cardiovascular pathological condition is heart injury from ischemia and reperfusion that bring about inflammation and necrosis. It was shown that proliferative nature of PAR2 agonists help in healing. PAR1 overexpression from disease status increases arterial wall thickening. This is due to mitogenic and proinflammatory effect of PAR1. Blocking PAR1 with antibody was shown to attenuate the problem. Cardiac hypertrophy is induced by PAR1 and 2 agonists, and this can be applied in myocardial infraction, in which rapid tissue repair and regeneration is required. A number of cardiac pathological conditions also up- or down-regulate PAR expressions. PAR2 expression is increased in umbilical vein endothelial cells under influence of inflammatory interleukins in Pre-eclampsia (pregnancy-associated hypertension).
Conclusion
Inflammatory and tissue damaging cardiovascular diseases induce PAR mediated vaso-relaxation, hypotension and proliferation. The immune and coagulatory systems are also PAR regulated. It is possible to apply PAR agonists for treatment of ischemic heart and thrombosis and research on receptor protease interaction would help designing appropriate cardiovascular agonistic and antagonistic drugs.
Reference
Cicala, C. 2002, ‘Protease activated receptor 2 and the cardiovascular system’, British Journal of Pharmacology, vol. 135, pp. 14-20.
Hirano, K. 2007, ‘The Roles of Proteinase-Activated Receptors in the Vascular Physiology and Pathophysiology’, Arteriosclerosis, Thrombosis, and Vascular Biology, vol. 27, pp. 27-36.
Leger, A.J., Covic, L. & Kuliopulos, A. 2006, ‘Protease-Activated Receptors in Cardiovascular Diseases’, Circulation, vol. 114, pp. 1070-1077.
Ossovskaya, V.S. & Bnnnett, N.W. 2004, ‘Protease-Activated Receptors: Contribution to Physiology and Disease’, Physiological Reviews, vol. 84, pp. 579-621.
Shpacovitch, V., Feld, M., Hollenberg, M.D., Luger, T.A. & Steinhoff, M. 2008, ’Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity’, Journal of Leukocyte Biology, vol. 83, pp. 1309-1322.
Traynelis, S.F. & Trejo, J. 2007, ’Protease-activated receptor signaling: new roles and regulatory mechanisms’, Current Opinion in Hematology, vol. 14, pp. 230–235.
Wilson, B.J., Harada, R., LeDuy, L., Hollenberg, M.D. & Nepveu, A. 2009, ‘CUX1 Transcription Factor Is a Downstream Effector of the Proteinase-activated Receptor 2 (PAR2)’, The Journal of Biological Chemistry, vol. 284, no. 1, pp. 36–45.