Introduction
Preface
The pharmaceutical industry is a complex, potent, and greatly globalized industry. It allocates the majority of its resources to the process of drug discovery, manufacturing, marketing, and logistics (Halliday et al. 1997).
It needs high research and development (R&D) expenditures and extensive regulation of its products compared with other manufacturing sectors (Micheal et al. 1999).
The pharmaceutical industry is one of the main industries in the world as more countries develop and promote constantly. It is a strategic activity as it concerns people’s well-being and protects public health.
The research is divided into five main parts, the first part contains an introduction includes Research problem, Research questions, Research objectives, and Research value. The second part contains a literature review provides an overview of the pharmaceutical Industry in Saudi Arabia with a focus on the components that are relevant to the competitiveness in this industry and factors affected it. This section also presents Porter´s theory with its five forces of competitiveness.
The research methodology was introduced in section three, which includes the target population, sample population, data collection methods and statistical treatment used. Part four displays the study results, while the fifth part concerned on the important recommendations and the Scientific Implications related to the study.
Research problem
Saudi Arabia is determined to become a regional leader in life science industries especially pharmaceuticals industries. Moreover, Saudi Arabia offers a variety of impressive opportunities and investment including specific areas such as the efforts of The Saudi Government for expanding the industry’s regulatory framework in addition to strengthening its intellectual property law regime to foster greater innovation and domestic manufacturing for this sector.
The pharmaceutical market in Saudi Arabia is considered one of the largest and the richest markets in the Middle East and North Africa. The Saudi Arabia’s citizens are the largest consumers of pharmaceutical products in the Gulf region, as the consumption of medicines is estimated to be $52 per capita, compared to about $20 in the rest of the Arab world (Issa et al. 2009). In 2014 the total pharmaceutical expenditure (TPE) in the Saudi market reached about 5 billion dollars (U.S Commercial Service Healthcare Technologies Resource Guide 2015).
This growth in Saudi Arabia pharmaceutical industry can be seen in the wide product lines; ranging from pure generics to branded generics which enables the consumer to exercise choices (Bawazir 2013), Furthermore, Saudi pharmaceutical market goes over the other Arabian Gulf market in the number of generic brand manufacturers with local pharmaceutical industry.
The local industry for drugs in Saudi Arabia covers between 16 and 20 percent of the domestic market needs, and the industry imported the rest of its needs. In 2012 Saudi Arabia imported drugs of about 10.8 billion Saudi riyals (2.88 billion dollars) (Bawazir 2013).
Given the importance of the pharmaceutical industry in the Kingdom with the presence of 33 pharmaceutical companies manufacture medicines and a big number of distributors for many international companies, it is important to study the attractiveness and competitiveness of this industry, whereas Porter’s model is applied to determine the intensity of competition within the industry and to supports analysis of the driving forces in the market. Furthermore, it helps management to decide how to develop particular characteristics of the industry.
Research questions
The following research questions outline how the study will be focused:
Firstly, the author asked, is the pharmaceutical industry is economically promising, attractive and profitable industry in the Kingdom? Is it having intensive competition? How can we apply porter’s model to measure the competitiveness in pharmaceutical industry in Saudi Arabia? How can suppliers drive prices upwards? Is it possible for buyers to drive down prices? How easy for competitors to enter the market? Does the Saudi pharmaceutical products compare favorably to other possible substitute?
Is rivalry among competitor high? What are the characteristics of the Saudi pharmaceutical market? Which is the possibility that support or hinder the pharmaceutical industry? How to finding out the opportunity and threat to the pharmaceutical sector in Saudi Arabia? And finally, what is the role The Saudi Arabian government to support and encourage the Pharmaceutical Industry?
Research objectives
This study aims to:
- Describe the competitive factors in Saudi Arabia pharmaceutical industry using the theoretical models of porter.
- Outline the forces such as the economic and legal factors that affect the Saudi Pharmaceutical Market.
- Highlight the role played by the Saudi government in an attemptto raise the level of national pharmacological security, and then upgrading the level of human development indicators of the Saudi citizen.
- Complete integrated analysis of the drug manufacturing policies inSaudi Arabia that underline the problem of competitiveness with manufacture decision-making and drug policies.
Research value
The pharmaceutical sector is a knowledge-based manufacturing industry and an important part of the health care sector; it represented 25 percent of the total spending on Saudi Health Care expenditure, which estimated at 5 billion dollars. This make the Saudi pharmaceutical market as the richest in the Gulf region, and this raise the need to have specialized studies and analysis on Saudi pharmaceutical industry competition. This study takes its importance from the following considerations:
- Importance of pharmaceutical industry worldwide.
- Importance of pharmaceutical industry in Saudi Arabia.
- Importance of pharmaceutical industry structure analysis and how it affects competition and profitability.
- Importance of giving a scope on changing strategies related to market environment.
- Enrich the Arabic library.
Literature Review
Overview
In this chapter the relevant terminology has been defined, the theories and themes governing the competitiveness in pharmaceutical industry have been discussed keeping in mind the study objectives. The following literature review will provide an overview of this Industry in Saudi Arabia and the factors affected it. This chapter will proceed in three major sections. First, background about the pharmaceutical industry will be described. Second, shows the industry definition. Third, gives a glance on Global pharmaceutical industry. Fourth, discuss the pharmaceutical industry in Saudi Arabia and factors affected it. And finally presents Porter´s theory with its five forces of competitiveness
Industry Background
The pharmaceutical industry is one of the main industries in the world as more countries develop and promote constantly. It is a strategic activity as it concerns people’s well-being and protects public health.
The pharmaceutical market “has undergone fast, exceptional, tremendous and complex changes in the last several years” (Al-Hag 2013, p.3). The pharmaceutical industry is considered one of the most inventive, innovative and profitable industries. These features have led to categorize it as a high-tech industry (Kasapiand Mihiotis 2011). The pharmaceutical industry represents a considerable added value to the national economy through export or direct investment (Al-Hag 2013).
The importance of big changes in pharmaceutical industry should be considered due to intensity in Research and Development (R&D) activities, uncertainty in drug development process, lack of new products, rapid integration, rapid development of generic markets (KarhuandYla-Kojola 2010), and finally increased global competition and technological advances (McAdam & Barron 2002). Moreover, some unique characteristics such as high-regulated setting, the long development process, risky and high level of cost in research phase (Cardinal 2001) distinguish the pharmaceutical industry from other industries.
The rapid changes in consumer behavior led to the significant growth in demand for pharmaceutical products in the 1990s. The increase in demand led to the growth of the market size, which was characterized by the changing nature of competition and increased competitiveness (Hadzović 1997).
Industry Definition
The pharmaceutical industry is responsible for the development, production and marketing of medications (John L. McGuire et al., 2007). However, companies that operate in the industry are required to comply with the laws and regulations governing the production, distribution, and consumption of the products besides the “patenting, testing and ensuring the safety, efficacy, and marketing of drugs” (Harrigan 1984, p.2).
According to Lee, Ahmad, Ahmed and Nawaz (2010), drug companies operate like other companies by manufacturing products for profitable sales in order for them to survive and grow. However, they are different from some companies because the drug business is very risky. Ingelman-Sundberg, Oscarson and McLellan (1999), Ahuja and Scypinski (2010) and Harrigan (1984) argue that manufacturers require high capital investment for research and development to patent new drugs.
Because drugs are consumed by human beings, the process of researching, discovering, and developing new drugs is expensive and time consuming. That makes a small percentage of products to be eventually approved for human consumption. Most national governments appoint medical institutions or boards to evaluate and to approve new drugs before they can be marketed.
The total industry can broadly be classified into two categories.
These are:
- Patent Medicines
- Generic Medicines
According to Kheir et al. (2008), patented medicines are the products that are invented by the companies, who have their own research team working on their own laboratories. These companies range from very large multinationals to small management establishments (SMEs). These products are patented for many years to enjoy the monopoly market. After years of business, the formulation is sold in the market so that others can go into mass production.
Generic medicines are products that are produced by several companies using a business model aimed at the development of a medicine which is identical or equivalent to Patent Medicines under different brand names in mass scale. These are marketed as soon as the originator product encounters loss of exclusivity. The Generic medicines are sold at a much lower price than the original product.
The difference in business models between originator and generic companies is reflected in their cost structure. The manufacture cost products for generic companies account for the largest share of the costs, for originator companies’ research and development, marketing and sales together account for a much larger share of total costs than the manufacturing process.
Pharmaceutical companies commonly spend a large amount on advertising and marketing. Different advertising platforms are used to reach the customer including the mainstream media and healthcare journals. However, some countries encourage direct adverts to the consumer. Companies make direct marketing possible by employing and engaging the services of marketing representatives who have direct access to healthcare workers and physicians.
Global Pharmaceutical Industry
One of the most profitable industries for many decades has been the global pharmaceutical industry (Kola, Landis 2004), however, due to dynamic forces in the competitive as well as regulatory environment, the conditions of the industry have changed.
Some of the factors that have contributed in different ways to the nature of the industry has been the strong dependency on innovation, the high risks involved, and R&D, and the supply chain management activities (Jaberidoost et al 2013), cause to decrease the attractiveness of the pharmaceutical industry compare to other industries (Gassmann et al 2008). Studies show that new drugs are expensive and time consuming to develop and the underlying process is extremely risky. Studies to determine the cost of bringing a new drug into the market show that approximately $800 million must be incurred (DiMasi2002 & 2003).
Moreover, it is estimated that an average of 12 years would have been passed from the synthesis of the new active pharmaceutical materials to launch a new drug to the market (Matías‐Reche2010). Thereby, on average, out of every 10,000 ingredients synthesized in the laboratories, only one or two will successfully pass all the steps to become marketable medicines (Festel et al 2010). Meanwhile, international competitiveness is becoming more important for the pharmaceutical industry.
Increased competitiveness and the changing structure of competitors impact the strategic direction of the pharmaceutical companies in the world (Kesič 2009). On the other hand, companies try to increase the profitability of all phases of the value chain from primary discovery research to production phase and logistics as well as sales and marketing phases (Zarenzhad et al. 2014).
Sales revenues generate company profits, allowing for more research, which in turn might lead to another successful novel product (Conklin 2010). Creating a cycle, which is initiated whereby, profits from existing drug sales fund and marketing of future drugs (Eljelly 2004). The cycle may potentially be repeated for each new drug developed and marketed. That makes the pharmaceutical company’s research and development (R&D) infrastructure is a major contributing factor to its competitiveness in the industry (Michels and Jonnard, 1999).
Competition in the industry occurs within the sectors and among other sectors. In the brand name sector, brands point out to treat with certain drug via the competition on its safety, efficacy, and brand equity. However, manufacturers of generic drugs do not incur the initial research and development costs that are often very expensive. They merely compete only on the lower prices of their products (Morton 1999).
Relatively low costs allow the generic sector to compete on price by offering generic equivalent drugs at prices that are approximately 70% less than those of brand name drugs (Generic Pharmaceutical Association [GPA], 2011)
Saudi Pharmaceutical industry:
Historically, the first pharmaceutical factory was established in the Kingdom of Saudi Arabia in 1926 to manufacture Eucalyptus pills for the treatment of citizens and pilgrims from malaria, then a factory for typhoid and smallpox vaccines was established (Bawazer 2010).
In the mid-thirties, pharmaceutical trading was grown and emerged agencies and drug stores were selling pharmaceuticals in cities like Jeddah and Makkah, The first regulation of pharmaceuticals and herbal trading was issued in 1938. The name of Public Health Department was changed to the Ministry of Health in 1950 (Bawazer 2010).
In 1959 an important shifting point was founded in the history of pharmacy in Kingdom, through establishing the first college of pharmacy in King Saud University. During the years followed, the total growth in the health sector was increased and drug stores and pharmacies were spread in deferent areas (Bawazer 2010).
The Saudi pharmaceutical industry has been adapting itself more and more to the strategic market trends and demands. The Saudi Arabian pharmaceutical market is one of the largest in the Middle East and North Africa. Its expenditure in 2014 represented 25 percent of the total spending on health care which estimated at USD 20 billion (5 billion dollars), growing an average of 10 percent annually (U.S Commercial Service Healthcare Technologies Resource Guide 2015). It can be concluded that patients’ bargaining power is significant and the overall power of buyers is considerable.
The pharmaceutical industry in Saudi Arabia is one of the most successful business sectors. As the companies compete on products’ characteristics and tend to invest heavily into marketing activities to gain physicians’/patients’ loyalty (McGuire, 2007).
Saudi Arabia is considered as one of the best countries that protect the property rights of the pharmaceutical industries leading to greater openness to the international market. Saudi drug market is characterized by strong presence of major international companies. They enter the market through strategic partnerships with local companies. The market characterized by a dynamic pricing system responsive to changes, balancing the reasonable price and the availability of medicines (Bawazer, 2013).
As Figure (1) indicates, six out of the top ten pharmaceutical companies in the Saudi Market are international companies (IMS Health, 2010). The result was an increase in the scope of competition from the local to the global level. The leading foreign firms in Saudi Arabia include; GlaxoSmithKline (UK), Pfizer, Novartis, Astra, MSD and Janssen, etc. While, the Leading domestic manufactures in Saudi Arabia are; SPIMACO, Tabuk Pharmaceuticals Company, Jamjoom Pharma, Al-Jazira Pharma, Riyadh Pharma, and Saja Pharmaceuticals. In Saudi Arabia, “foreign pharmaceutical companies don’t have a legal entity as they must work through local Saudi agents” (Al Shaikh 2011, p.29).
The private pharmacy sector tends to support branded pharmaceutical industry as it is marked by tight price controls (Al Shaikh et al, 2011)
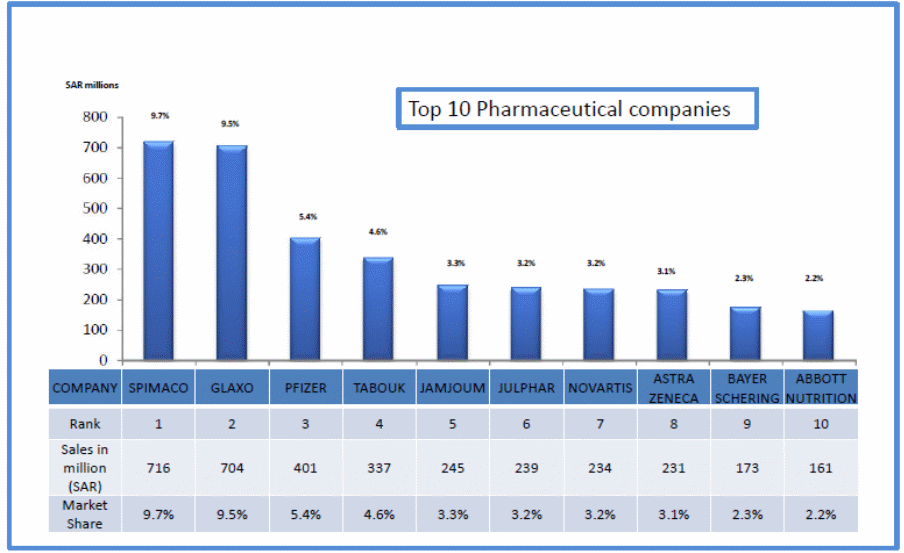
Building a life sciences industry in Saudi Arabia is one of the government’s top economic priorities. In addition to transforming the economy and its infrastructure to support knowledge-based industries, the government is supporting the sector’s development through a variety of direct and complementary investments. According to the “Saudi Pharmaceutical Market is fragmented among 271 companies (IMS Health, 2010, p.7), Saudi pharmaceutical manufacturers “are mainly involved in secondary manufacturing, through the use of active ingredients of a pharmaceutical drug due to the increasing number of primary products losing patent protection worldwide” (Business Monitor International 2010, p.19).
The domestic production in Saudi Arabia supplies only 15 % of the market need and 85% of the market need is imported. (85% of its output is exported to the other GCC countries). Therefore, it imports a large amount of semi-finished medicaments, some of which are then relabeled, repackaged and exported (Issa et al. 2009).
According to Enright (2003) and Bawazer (2013), Saudi Arabia consumes about 80 percent of the innovative medicines and the remaining 20 percent goes for the generic medicine. This gives great opportunities for the development of investment in the field of medicine in Saudi Arabia. Fina and Rugman (1996) and Frank (2007) have pointed out that the pharmaceutical industry in Saudi Arabia has shown a quick development of the world generic markets and generic drug manufacturers rely on government substitution laws to take sales away from branded counterparts. Government policies are biased in favor of domestic producers, providing them exemptions, including interest-free funding, subsidized utility charges, and no import duties on raw materials and intermediate products (U.S Commercial Service Healthcare Technologies Resource Guide 2015).
At present, there are about 30 manufacturing facilities in the Kingdom that produce the following products: Augumentin, Viagra, Snafi, Lipitor, Rofenac, Klavox, Zithromax, Nexium, Dermovet, etc. It is important to declare that generic drugs are gaining market share in the Saudi pharmaceutical market, since they are considerably cheaper, resulting in the Saudi pharmaceutical industry being more competitive (Al Sheikh et al. 2011).
(Rashid 2014) stated that, unfortunately no factory not only in the Kingdom, but also in all Arab countries licensed for drug industry from the original company. The role of manufacturer in our region is limited to the manufacture of medicines that have passed the period of protection (10 years). But that does not mean it is a weak manufacturer, because there is a high demand on these drugs as it reduces numerous amounts of money that Saudi Government pays for Government Health Services (Fung et al. 2001). It also relieves the burden of importing medicine with original high cost and replaces it with generic medicines of the least cost.
(AL-Abdulkarim 2014) said that, the Saudi market is highly competitive, open for all companies and there is a lack of control over the prescribed medication by physicians thus makes the national firms find it difficult to compete. He added that, the lack of the information in the pharmaceutical industry sector makes investors fear from pumping massive money without having sufficient information about the market, as the investment in this sector needs huge capital.
Al-Abdulkarim presented a number of proposals to support the local pharmaceutical industry like increasing the Saudization of more than 40% at least with recruiting well-trained Saudis. Such support will stimulate international companies to move there manufacturing to the Kingdom.
Economic factor that affect Saudi pharmaceutical industry
According to the Saudi Arabian General Investment Authority, Saudi Economy “is ranked among the top ten most competitive economies because of the oil and centralised controls of major economic investments and related activities” (Business Monitor International 2010, p.3)..The “Saudi Pharmaceutical market is the largest among the Gulf Cooperation Council Countries with an estimated double-digit annual growth till 2019” (Business Monitor International 2010, p.5). Such a high growth rate should signify a striking chance to foreign pharmaceutical companies to invest their job in the kingdom.
The Food and Drug Administration has estimated the local pharmaceutical industries growth rate in Saudi Arabia has been 40 percent in last seven years, and happens according to increasing number of local factories. The growth has triggered an increase in the application of intellectual property laws on pharmaceuticals industry.
Drugs manufactured in Saudi Arabia are within 33 local factories, two of them for veterinary medicines. The number of factories specialized in pharmaceutical industry in Saudi Arabia is expected to be 40 pharmaceutical factories by 2020, which will increase the ratio of locally manufactured products to between 35 and 40 percent.
Bawazer (2013) stated that, there are great opportunities for the development of investment in the field of drugs; by direction the global companies to build their factories in King Abdullah economic city in Saudi Arabia (western zone) beside the presence of a large number of companies in the Saudi market.
Increasing health insurance is considered as a factor that helps in increasing the consumption of drugs indirectly, particularly for people with chronic diseases (Bawazer 2013).
With enhancing the technology level thus will create a good media for market competition. As most of the “local Saudi companies don’t have the know-how of manufacturing high technological products like biotechnology products and anti-cancer therapy” (Business Monitor International 2010, p.12).
Industrial cities commission « Modon » in Saudi Arabia is planning to establish the first pharmaceutical complex in the region, aimed to create an appropriate environment for the industry through the spaces, technology and integrated services in supply chain opportunities from raw materials, production and packaging, or support services thus enhance the competiveness in the industry (Bawazer 2013).
Legal factor that affect Saudi pharmaceutical industry
Legal factors are related to the legal environment in which firms operate. Law is the supreme command of any nation Health Laws included (Palmer, 2004). Companies need to be aware of the risks involved in the legal environment and are often advisable to have a reliable local partner and an attorney who can deal with the legal issues (Morrison 2009).
In Saudi Arabia, there are legal provisions establishing the powers and responsibilities of the Medicines Regulatory Authority (MRA). Legal provisions exist requiring authorization to import medicines. Laws exist allow the sampling of imported products for testing. Regulations or laws exist to allow for inspection of imported pharmaceutical products at authorized ports of entry (Issa et al. 2009).
In Saudi Arabia, there are legal or regulatory provisions affecting pricing of medicines. These provisions are aimed at the level of manufacturers, wholesalers and retailers (Alakloby 2005). They affect: Innovated Patented Products, Generic Products, Under License Locally Manufactured Products, Products with Different Package Sizes or Strengths, Products that have Specific Advantages, and fixed Combination Drug Product. Legal provisions exist requiring manufacturers to be licensed (Alpen Capital 2013).
AL-Mishaal (2014) emphasized that the Saudi government (Saudi food and drug authority) aims to support localization of institutions and private sector companies, especially in the drug sector and medical devices to be in competition with the international companies. Moreover, the Saudi food and drug authority work to balance the availability of medication and high prices, so it established a (competent committee of drug pricing agency) with a specific rule like the cost of manufacturing and economic indicators.
Also, the political environment in Saudi Arabia has a great influence on management and leadership of pharmaceutical company (Al-Wazaify et al. 2006). Political environments refer to public institutions (such as the government, government agencies, and government-owned businesses) and non-public institutions (such as environmental and other special interest groups that represent specific individuals or groups) (Baron 1993).
According to Asiri (2011, p.3) and the Business Monitor International 2010, p.5), “the political system in Saudi Arabia is relatively stable, which could represent a motive for international pharmaceutical companies to invest in the kingdom”.
Porter’s five force analysis
Michael Porter’s initial thoughts on how competitive forces shape strategy were formed in1980 and later expanded upon in 2008 (Kotler & Armstrong 2010). These forces are used to better understand the complexities of the competitive environment and to analyze profitability of an industry. Porter created a “five forces” model that outlines the competitive forces.
This section briefly presents Porter´s theory with a focus on the components that are relevant to the competitiveness in pharmaceutical industry in Saudi Arabia. That aims to provide a strategic framework to analyze industry structure and how it affects competition and profitability. This type of analysis enables firms to be prepared for, and take advantage of challenges in order to survive in a competitive industry.
Porter (2008) made a critical observation by not only understanding the underpinnings of competition and the root causes of profitability, but also how their competitors will shape the amount of profit they can generate. According to Kulkarni (2004, p.9) and Weir (2013, p.13), “Porter’s Five Forces Analysis is an important tool for assessing the potential of profitability of a business”. Understanding of these forces helps a firm through increasing its knowledge of its own industry, be better prepared to face any challenges and furthermore it increases long-run success.
According to Porter (1985, p.11), the “five forces model provides the basis for structural analysis of industries with a powerful tool for understanding where power lies in a business situation”. They determine the profitability and the degree of attractiveness of a given industry and constitute strong threats to a company’s profitability (Baker 1992 & Bradley 1995).
Grundy (2006) developed advantages which significantly act with the porter model acting on the business strategy which are: emphasizes the importance of negotiating power, bargaining arrangements in determining relative market attractiveness, how competitive rivalry is very much function than other four forces and combines input-output analysis of a specific industry with industry boundaries via entry barriers and substitutes.
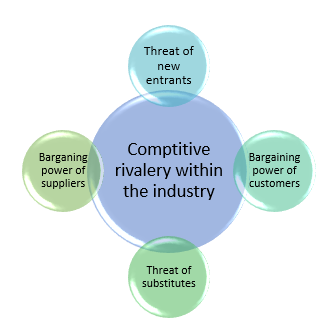
As shown in Figure 2, Porter’s 5 Forces are clearly illustrated. Recklies (2001, p11) and Lee et al. (2010, p5) indicated that “Porter’s forces determine the intensity of competition, hence the profitability and attractiveness of an industry”. The “objective of this model is to modify these competitive forces in a way that improves the position of the industry and provides an analytical view of the forces in the market” (Bingham & Eisenhardt 2008, p.23). Furthermore, it helps management to decide how to develop particular characteristics of their industry.
Rivalry within the Industry
Grant (2010) considered the force that commonly gains the most attention in the industry regarding the existing competitors. The rivalry among existing competitors is huge if the competitors are numerous. Rivalry occurs because one or more competitors either feels the pressure or sees the opportunity to improve its position (Cafferky, 2005). The intensity of rivalry can increase if the industry growth is slow, since more firms compete for the market shares. Competition within an industry can also be more intense if the barriers to exit are high (Bruzelius & Johansson 2012). The industry of rivalry depends on the barriers are associated with costs of leaving the industry, this obstacle implies how firms can be challenged to exit owing to the high costs (Shin 2001).
The number and diversity of competitors are factors that have effects on rivalry within the industry (Barney 2007). Moreover, it depends upon the high fixed costs, as the firm must produce its product at a high output level in order to lower per unit cost. This implies that a high level of production will urge the firm to compete for market share and this will cause an increase in rivalry (Miller 2002).
Threat of Substitute Products
In Porter’s model, threat of substitutes refers to a degree, to which products or services can satisfy the same needs of the local industry. Lowering the price of a product increases its performance in the market (Barney 2006). It has been established that substitute products influence the pricing plasticity of a product in the market and when substitutes are cheaper, the more of those products enter the market (Braithwaite 2013). With multiple alternatives and substitute products to select from, elasticity of demand occurs, which is an inhibiting factor of an increase in prices.
Substitute products have an adverse effect on the profits of a company that sells patented products. This rivalry can exist among individual or whole industry. Competition from similar substitutes to product constrains the possibility of price with large margins. Existence of similar, suitable and affordable substitute will encourage consumer to shift to the cheaper price if they are interested in the price. On the contrary if the product is unique or novel, the consumer will charge even if it is at high price. In the other hand the lack of substitutes will decrease the consumer sensitivity to the price (Bruzelius & Johansson 2012).
(Charles and Warren 2006) detected that; the existence of close substitute products increases the propensity of customers to switch to alternatives in response to price increases (high elasticity of demand).
Barriers to New Entrants / Threat of Entry
Threat of New Entrants refers to the degree to which new competitors can join an industry (Porter 1985). New entrants result in to profitable markets that yield high return. The profit rate will fall towards a competitive level (perfect competition). Profits can be used to determine the nature of the market and the competition experienced.
This force concerns with barriers to entry benefit especially the incumbent firms, but if an entrant can overcome these barriers they potentially create a threat to the incumbent firms (Bruzelius & Johansson 2012).
The established firms have differentiated from one another via construction of a strong brand name with loyal customers, making it hard for new companies to build up a brand name (Carter 2005).
A new entrant is mainly faced with entry costs. If an entrant would like to enter a market it can be necessary to invest in a large amount of advertising and R&D. That will prevent new entrants with weak finances from entering an industry (Grant 2010).
Bargaining Power of Buyers
“Buyers” refers to both a firm’s retailers and consumers. Both of them are price-sensitive if they buy an unnecessary good. Retailers can gain bargaining power over manufacturers if they can influence consumers downstream using either advertising or with the help of setting final prices. That gives the retailers a possibility for negotiation with an upstream firm, which the consumers do not have (Bruzelius & Johansson 2012).
When buyers have details about the cost of industry and the price levels, the buyers bargaining power will increase. While, as soon as buyers have access to information, they can use their power to play off firms against each other by demanding lower prices, a higher quality and a higher level of service and thus increase competition within an industry (Cabral 2000).
The price elasticity of demand is high, and the level of consumption is highly correlated to changes in price when buyers are price-sensitive, thus an increase in the price level of certain good will lead to a decrease in revenues due to decreased sales (Bruzelius & Johansson 2012).
Also buyers can threaten as they can expand their own production vertically and start producing their supplier’s products themselves, if the supplier does not meet their demands (Grant 2010).
In the healthcare market segment buyers would include the patients, insurance companies, and distributors, pharmacies, and government’s health institutions.
Buyers do not proof a big threat to the pharmaceutical industry because firms spend most of their research and development on new patent drugs (Paul et al. 2010). Since the industry has many buyers and competition normally occurs among consumers (e.g. competition among hospitals or drug stores), the power of buyers in terms of the number of buyers in the industry is relatively small (Kasapi and Mihiotis 2011).
Bargaining Power of Suppliers
The bargaining power of Suppliers in the industries appears when they are able to charge higher prices or limiting quality or service. Companies within an industry frequently utilize the suppliers for their production so they face suppliers with a large bargaining power.
As a supplier, setting higher prices are a way of capturing a consumer (industry) surplus and making it a producer (supplier) surplus, which leads to a decrease in social welfare since the quantity produced, will be less than in a perfect competition. This could lead to squeeze-out of firms that cannot deal with the increased costs via raising their own prices, especially if the customers are highly price-sensitive (Bruzelius & Johansson 2012).
Lynch (2006) emphasized that; the bargaining power of suppliers refers to the ability of suppliers to influence cost, availability, and quality of input materials to firms within the industry.
A supplier of the pharmaceutical industry could be anyone from the provider of raw materials, labor forces and the makers of active ingredients, packaging, distributors and consulting agencies. (Weir 2013)
Research Methodology
Research method
This section adopts an original survey-based quantitative approach (primary data) to examine SPI global competitiveness (Gill & Johnson 2010). Taking into account that the paper depends on the opinion of “industry experts”, however, the response bias is minimized.
Measurement tool
A questionnaire consisted of six main sections with a total number of 28 questions, which used to collect quantitative data. These sections included questions related bibliographical data (3 questions) the bargaining power of suppliers (5 questions), the bargaining power of buyers (5 questions), the threat of entry (7 questions), the threat of substitutes (3 questions) and the rivalry among competitors (5 questions).
The Likert scale may be conducted from a scale one to five.
- strongly disagree,
- disagree,
- neutral,
- agree,
- strongly agree.
A Likert scale is a type of research method tool frequently used in research surveys. It allows respondents to indicate how much they agree with the statement that is posed. This type of scale measures how much the respondent is in agreement with the question. For instance, a respondent could be in total agreement, feels strongly oppose to the statement, or the respondent is sitting on the fence (in between both sides).
Sample population
A questionnaire survey has been distributed by hand to 28 experts, who were affiliated to four pharmaceutical companies, 24 responses were received from participants with 85.7% response rate.
Statistical techniques
Survey results were analysed using SPSS program to calculate Percentage, Number of frequencies, Mean, Std. Deviation, T-test, p-value and t-test of mean difference to represent the comparison based on respondents’ position experience and degree (Lazar, Feng & Hochheiser 2010).
T-test is a parametric test, which reflects how different the sample mean value for a specific value that the researcher wants to test, (Test Value = 3). This test reflects the extent of the significant responses on the total sample questions from the questionnaire.
The study explains the relationship of one variable with another through the research elements. After the questionnaire is completed, each item is then analysed separately to create a score for each item, and as a group of items and then in overall industry. Analysis and comparison are presented in form of published studies.
Results and Analysis
Sample Description
The sample size consisted of experts who have 5 to 10 years’ experience working as managers in marketing, financial, operations, sales, quality assurance and quality control departments. The participants were distributed as follows: 9 of the experts were top level managers (37.5%) and 15 were middle level managers (62.5%) as shown in table (1). Table (2) shows the distribution of the population used in the study, which shows that 8 of the experts have 5 to 10 years of experience (34.8%), 16 of the experts have more than 10 years of experience in the area of specialisation, making 65.2%. The levels of education of the experts were distributed as follows: 11 of them have undergraduate degrees (47.8%) and 12-hold graduate degree (52.2%) as shown in Table (3).
Table 1: Sample description based on positions.
Table 2: Sample description based on experience.
Table 3: Sample description based on degree.
Data Analysis
Overall analysis of the pharmaceutical industry in SA
Table 4: Total Porter’s Analysis.
Table 16 shows a summary of the overall effects of Porter’s forces on the competitive advantage of companies operating in the pharmaceutical industry in Saudi Arabia to be favourable based on the outcome of the responses from 24 respondents, with a mean of 3.1352 and a standard deviation of.28636.
Table 5 attractiveness of the pharmaceutical industry.
Porter’s five forces Analysis
Based on the results of variables analysis found in Table 5, the score on three variables was less than 0.05 (p<0.05), thus, we reject the null hypothesis. The results show that the participants support the approval of the community, a position that is supported by the mean value of the five axes because it is different from zero, i.e. they are moral values hypothesis. The p<0.05 shows that the rejection of the null hypothesis is reliable because the possibility of making an error in decision making is equal or less than 5% against a 95% confidence interval. Typically, the three variables include the power of buyer (p<.009), power of supplier (p<.006), and competitors rivalry (p<.002).
When compared with the p<0.05, the results were deemed to be in favor of the companies operating in the pharmaceutical industry in the context of the three forces. However, when the p<0.05 was used to evaluate results of the threat of new entrants into the industry scored 0.173 and that of the threat of substitutes products had a p-value of.524. In both cases, the p-values are more than 0.05, which shows a moderate effect of the forces on the on the competitive advantage of the pharmaceutical industry in Saudi Arabia.
One-Sample Statistics
Table 5: Porter’s 5 forces analysis.
Bargaining Power of Suppliers Analysis
Through the analysis of the statements in table 4, respondents showed that it is difficult for SPI suppliers to enter the industry business, sell directly to the customers, and become a direct competitor because the mean score for the related variable is 3.79 (M = 3.79).
Also, the results showed that the input products purchased by the companies operating in the pharmaceutical industry from the supplier side represent a high proportion of the volume of business, which is represented by a means score of 3.67 (M=3.67). It is evident that the industry has a large number suppliers who provide inputs for companies manufacturing pharmaceutical products, which is represented by a statistic mean of 3.50 (M = 3.50). A mean of 2.75 shows that SPI companies intending to switch to substitute products from the supplier side find it difficult because of the challenges that are difficult to overcome (M = 2.75).
Table 4: Bargaining Power of Suppliers analysis.
- 1. The researcher established that the statistic mean for the power of suppliers was M = 3.50 and standard deviation was 1.063, which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to number of potential input suppliers. The results show that the number of suppliers in the industry is small and that places the industry players at a competitive advantage increasing profitability and specialization.
- 2. The researcher finds that the mean is M = 2.75 and standard deviation is 1.294 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to transfer from a supplier to another. It shows that the level of competition and specialization in the industry is low among the pharmaceutical industry players in Saudi Arabia. The moderate competitive advantage of the companies in enjoying supplying raw materials that are used in the pharmaceutical industry is related to the low levels of concentration and market structure of the suppliers and consumer organisations.
- 3. The researcher finds that the mean is M = 3.67 and standard deviation is 1.007 which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to the size of purchases of pharmaceutical industry companies from suppliers.
- 4. The researcher finds that the mean is M = 3.79 and standard deviation is.833 which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to the difficulty for suppliers to enter the pharmaceutical industry and become direct competitors. The rational is that the industry structure, portfolio experience, pricing strategies and the size of the market are some of the underlying factors that have created the impact on the mean and standard deviation.
- 5. The researcher finds that the mean is M = 2.92 and standard deviation is 1.060 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to partnership relation between suppliers and pharmaceutical companies. Here, partnership is a critical component in the pharmaceutical industry because of the economic consequences and that it is difficult to exert any controls on the suppliers. In addition, each supplier has it’s a customised business relationship with customers with an underlying unique business logic and relationships.
The vision can be viewed more clearly in figure (1) where it gives an indication of the acceptance of the levels related to (Bargaining power of suppliers).
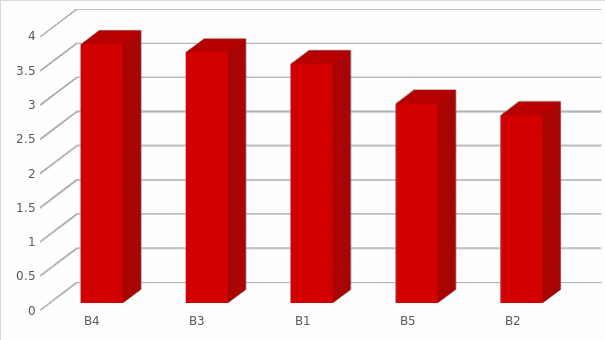
As shown in figure 1, the competitive advantage of suppliers is affected by a small or low presence of competitors willing to invest in the pharmaceutical industry. In general, the power of suppliers is classified as low for companies interested to invest in the pharmaceutical industry because the p-values are below the significant significance level when evaluated against the mean values of the responses. In addition, the raw materials or products used in the pharmaceutical industry are deemed to be simple, not differentiated, and without any specialization, making the switching costs low for the companies operating in the industry.
Power of Buyers Analysis
The results based on responses from 24 participants showed a standard deviation of 0.963 and a std. error of the mean of 0.197, showing that if a company loses buyers, the effects could be small because of big the size of the market. The mean of the number of buyers to be 3.17 (M=3.17), which shows that the power of the buyers is moderate and can influence the competitive advantage of a firm depending on the level of attraction to buy a company’s products.
A mean of 3.13 (M=3.13) in table 5 shows that the percentage income spent on pharmaceutical products is small, which show a moderate effect on the competitive advantage of the firms. A mean of 2.79 (M=2.79) shows that most of the companies have “locked in” customers who cannot shift and start consuming products from other companies. However, the number of buyers interested in purchasing pharmaceutical products is 3.96 (M=3.96) and a mean of 3.38 showing that the effects of buying the products from the companies is moderate and that stimulates a competitive advantage for the firms.
Table 5: Power of buyers’ analysis.
- The response from participants of the statement “There are a large number of buyers from Saudi pharmaceutical companies, such that losing a buyer isn’t critical to their success” shows the following statistics:
- The researcher finds that the mean is M = 3.17 and standard deviation is.963 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to number of buyers. This is an indicator of a dynamically growing market and the economy of suppliers of raw materials for the industry. Typically, the volume of business, product performance and quality, and how the relationships fit into the operations and strategy of the buying companies determine the extent of the competitive advantage.
- The response from participants of the statement “expenses on products of the pharmaceutical companies in SA represent a small portion of the buyers’ total expenses ” shows the following statistics:
- The researcher finds that the mean is M = 3.13 and standard deviation is.992 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to buyers’ total expenses on products of the pharmaceutical companies. Here, the market power of the firms that operate in the industry is moderate because the effects of increasing the price of products above the marginal costs on the purchasing behavior of customers is low, showing a low level of concentration of other firms in the industry. Typically, an increase in marginal costs cannot have a downward slope of the demand curve because of the low concentration and competition from other companies.
- The response from participants of statement “products of the pharmaceutical companies in SA are unique, which makes it difficult for a buyer to transfer from their products to competitors’ products “. The results from the participants show the following statistics:
- The researcher finds that the mean is M = 2.79 and standard deviation is 1.103 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to difficulty for buyers to transfer from Saudi Pharmaceutical products to competitors’ products. Here, the high switching costs have a significant positive effect on all players in the industry and facilitates higher marginal profits because most of the customers get ‘locked in’ into consuming products sold by specific pharmaceutical companies.
- The response from participants of statement “A large sales percentage of pharmaceutical companies in SA go usually too few numbers of buyers “. The results from the participants show the following statistics:
- The researcher finds that the mean is M = 3.96 and standard deviation is.955 which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to percentage of pharmaceutical companies’ sales that goes to buyers.
- The response from participants of statement “the government is the major buyer from the pharmaceutical companies in SA “. The results from the participants show the following statistics:
- The researcher finds that the mean is M = 3.38 and standard deviation is 1.209 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to major buyer from the pharmaceutical companies in SA. The inclusion of the government in purchasing the pharmaceutical is a clear evidence of the value of the industry both to the consumer and to those companies that are major players in the industry.
One-Sample Test
Table 6: Power of buyer’s statistics.
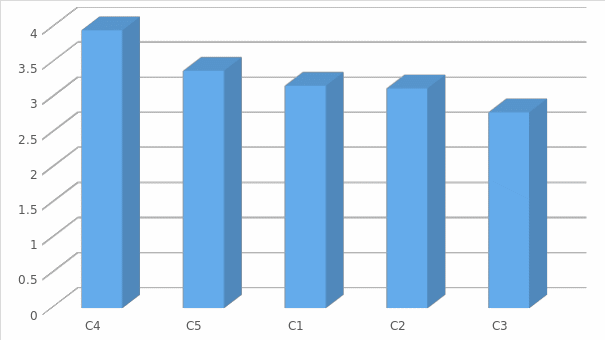
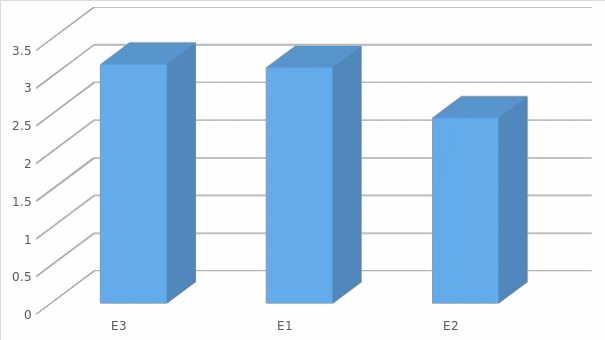
The vision can be viewed more clearly in figure (2) where it gives an indication of the acceptance of the levels related to (Bargaining power of buyers).
The one sample test on the power of the buyer shows that the power of the t-statistic is dependent on values that vary between 1.519 and -.926. The t-statistic shows that some of the variations that exist among the variables that were investigated provide a strong indication of the effects the companies have when evaluated on Porter’s five forces model.
Threat of Entry Analysis
The results tabulated on table 7 shows that new companies entering the market face strong resistance from those that have been in the market because, the mean response on differentiated products is 3.17 (M=3.17), which shows a moderate effect because most of the customers are loyal to their brands. Because of that, the mean response to the ease with which to establish a pharmaceutical company in Saudi Arabia is 2.75 (M=2.75), which shows a strong resistance to the entry of differentiated.
The factors such as establishing a new distribution channel of 2.96 (M=2.96), acquiring new inputs with a mean of 2.92 (M=2.92), regulatory restrictions of a mean of (M=2.42), investor reactions (M=2.38), and a mean of 3.13 (M=3.13), which shows how difficult it is for a new entrant to reach a breakeven point. An overall view is that it is difficult for new entrants to penetrate the market.
One-Sample Statistics
Table 7: Threat of Entry analysis.
- The response from participants of the statement “Products of pharmaceutical companies in SA are differentiated and customers are loyal to their brands” shows the following statistics:
- The researcher finds that the mean is M = 3.17 and standard deviation is.963, which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to customer’s loyalty to their brands.
- The response from participants of the statement ” It is not easy to establish a pharmaceutical company in SA because of the high start-up costs ” shows the following statistics:
- The researcher finds that the mean is M = 2.75 and standard deviation is.989 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to the start-up costs for establishing a pharmaceutical company in SA.
- The response from participants of statement ” A new competitor in pharmaceutical industry in SA will have difficulty acquiring/obtaining channels of distribution “. The results from the participants show the following statistics:
- The researcher finds that the mean is M = 2.96 and standard deviation is.955 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to difficulty for a new competitor in Saudi pharmaceutical industry acquiring/obtaining channels of distribution.
- The response from participants of statement ” A new competitor in pharmaceutical industry in SA have difficulty acquiring/obtaining needed inputs to compete efficiently “. The results from the participants show the following statistics:
- The researcher finds that the mean is M = 2.92 and standard deviation is 1.213 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to difficulty for a new competitor in Saudi pharmaceutical industry acquiring/obtaining needed inputs to compete efficiently.
- The response from participants of statement ” The government of SA puts restricts regulations to establish a pharmaceutical company which makes it difficult to go into this business “. The result from the participants shows the following statistics:
- The researcher finds that the mean is M = 2.42 and standard deviation is 1.176 which indicates that Saudi Pharmaceutical Industry has unfavourable advantage related to the government regulations to establish a pharmaceutical company.
- The response from participants of statement “Investors will hesitate to establish a new pharmaceutical company in SA because of possible reaction and retaliation from the existing companies “. The result from the participants shows the following statistics:
- The researcher finds that the mean is M = 2.38 and standard deviation is.824 which indicates that Saudi Pharmaceutical Industry has unfavourable advantage related to investors attitude to establish a new pharmaceutical company in SA in relation to possible reaction and retaliation from the existing companies.
- The response from participants of statement “Investors will hesitate to establish a new pharmaceutical company in SA because of difficulty of reaching the breakeven and economy of scale “. The result from the participants shows the following statistics:
- The researcher finds that the mean is M = 3.13 and standard deviation is 1.227 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to investors attitude to establish a new pharmaceutical company in SA in relation to difficulty of reaching the breakeven and economy of scale.
One-Sample Test
Table 8: Threat of Entry statistics.
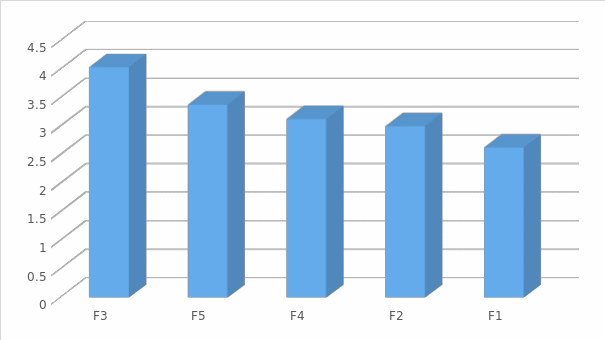
The vision can be viewed more clearly in figure (3) where it gives an indication of the acceptance of the levels related to (Threat of Entry).
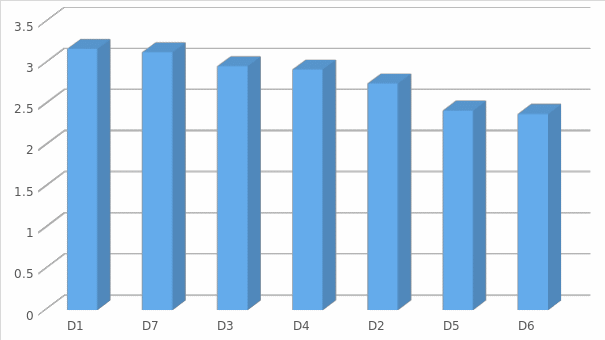
Threat of Substitute Analysis
The results show that consumer and pharmaceutical companies prefer to consume or sell products made in Saudi Arabia other than products from other countries and the responses showed a mean of 3.13 (M=3.13). The effects are moderate, which shows that consumers and companies still embrace products from foreign countries. A mean of 2.46 (M=2.46) shows that consumers find it difficult to switch to new products, which makes it unfavorable for new substitute products. Pricing is a strong factor that discourages the entry of substitute products because prices are kept as low as possible based on a mean response of 3.17 (M=3.17). However, the effect is moderate because highly priced products can still be bought in SA.
One-Sample Statistics
Table 9: Threat of substitute analysis.
The response from participants of the statement “Saudi pharmaceutical industry company’s products are more favourable comparing to other possible substitutes” shows the following statistics:
The researcher finds that the mean is M = 3.13 and standard deviation is.850 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to product preference comparing to other possible substitutes.
The response from participants of the statement “It is costly for customers of pharmaceutical companies in SA to switch to substitute products” shows the following statistics:
The researcher finds that the mean is M = 2.46 and standard deviation is 1.103 which indicates that Saudi Pharmaceutical Industry has unfavourable advantage towards customers switching from its products to substitutes.
The response from participants of statement ” Prices of products provided by pharmaceutical companies in SA are not high to push for arisen of new substitutes “. The result from the participants shows the following statistics:
The researcher finds that the mean is M = 3.17and standard deviation is.963 which indicates that Saudi Pharmaceutical Industry has a moderate advantage towards Prices of SA pharmaceutical products and the emergence of new substitutes.
One-Sample Test
Table 10: Threat of substitute statistics.
The vision can be viewed more clearly in figure (4) where it gives an indication of the acceptance of the levels related to (Threat of substitute).
Analysis of questions related to Rivalry among competitors
One-Sample Statistics
Table 11: Rivalry among competitors analysis.
- The response from participants of the statement ” There is a small number of competitors in Saudi pharmaceutical industry (local or international companies export to it)” shows the following statistics:
- The researcher finds that the mean is M = 2.63 and standard deviation is 1.096 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to number of competitors.
- The response from participants of the statement “Competitors of the pharmaceutical industry are close with each other in market shares” shows the following statistics:
- The researcher finds that the mean is M = 3.00 and standard deviation is.780 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to Competitors market shares.
- The response from participants of statement “The Saudi pharmaceutical market is growing “. The result from the participants shows the following statistics:
- The researcher finds that the mean is M = 4.04 and standard deviation is.690 which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to market growth.
- The response from participants of statement “It is not easy for a current pharmaceutical company in SA to exit the industry because of the high investment in fixed assets” The result from the participants shows the following statistics:
- The researcher finds that the mean is M = 3.13 and standard deviation is 1.076 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to the exit from industry because of the high investment in fixed assets.
- The response from participants of statement “It is not easy for a current pharmaceutical company in SA to exit the industry because of the difficulty of firing Saudi employees” The result from the participants shows the following statistics:
- The researcher finds that the mean is M = 3.38 and standard deviation is 1.013 which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to the exit from industry because of the difficulty of firing Saudi employees.
One-Sample Test
Table 12: Rivalry among competitor’s statistics.
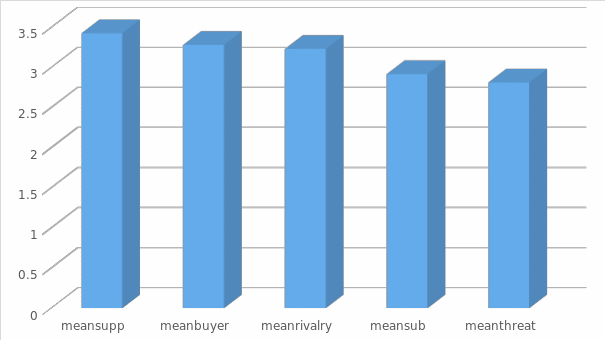
The vision can be viewed more clearly in figure (4) where it gives an indication of the acceptance of the levels related to (competitors’ rivalry).
Porter’s five forces Analysis
One-Sample Statistics
Table 13: Porter’s 5 forces analysis.
Based on the results of the analysis of data collected from the participants, (Table1) shows the following statistics:
- Mean power of suppliers is = 3.4271 and standard deviation is. 68556, which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to power of supplier.
- Mean power of buyers is = 3.2833 and standard deviation is .48960 which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to power of buyer.
- Mean power of threat of entry is = 2.8155 and standard deviation is .64231, which indicates that Saudi Pharmaceutical Industry has a moderate advantage related to threat of entry.
- Mean power of threat of substitute is = 2.9167 and standard deviation is .63131which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to threat of substitute.
- Mean power of rivalry among competitors = 3.2333 and standard deviation is .32123which indicates that Saudi Pharmaceutical Industry has a favourable advantage related to rivalry among competitors.
Table 14: Porter’s 5 forces statistics.
One-Sample Test
The vision can be viewed more clearly in figure (4) where it gives an indication of the acceptance of the levels related to (Porter’s 5 forces).
A Comparison between the opinion of the top management and middle management groups
A Comparison between the opinion of 5-10 years experience and more than 10 years’ experience groups:
Total Porter’s Analysis
Table 17: Total Porter’s Analysis.
Based on the results of the analysis of data collected from the participants, (Table 3) shows the following statistics:
Mean power of total porter’s 5 forces is = 3.1352 and standard deviation is .05845 and this indicate that Saudi pharmaceutical industry has a credit and favourable competitive advantage.
Table 17: Total Porter’s statistics.
Conclusion and Recommendations
Discussion & Analysis of findings
In practise, the responses from the 24 participants provide an overall view of the effects of Porter’s five forces on the competitive advantage of different organisations’ shareholding business operations in the pharmaceutical industry in Saudi Arabia. The results provide the foundation for shaping the competitive strategy for the firms. Here, the competitive advantage does not entirely depend on direct competition, but goes beyond the power of established firms.
The underlying factors of expansion and profitability with the inclusion of generic products, investments in marketing and advertising in healthcare journals, the supply side, economies of scale, value chain issues, and uniqueness of the pharmaceutical products, which are the same for all players in the market are important components that help to shape strategy. Typically, the rationale is that competition exists among the six major international companies and four local organisations, which make the top ten firms dominating the market.
Analytically, the score on the first three forces of the 24 respondents (competitors’ rivalry p<.002, power of buyer, p<.009, and power of supplier p<.006), are equal or slightly above the p-value of 0.05 based on a 95% confidence interval, providing statistical evidence, which shows that the forces have positively shaped the nature of competition among the 271 companies in the pharmaceutical industry in Saudi Arabia, with the top ten companies dominating the market being more strategically placed compared with other players.
A comparative analysis of the responses of those with 5-10 years’ experience in the industry show a favourable response for competitors rivalry, power of supplier (M=.76723, M=.52720), power of buyer (M=.41429, M=.37618), competitors rivalry (M=.44149, M=.18007) and threat of substitute (M=.86223, M=.38490) to be moderate. The threat of substitutes and threat of entry had moderate scores showing that such effects can be moderated by introducing partnership agreements with the 10 top companies and manufacturers.
The results show the magnitude of competition to be low among industry players. It is evident that the pricing mechanism adopted by the companies and the Government is determined by the variables such as the power of suppliers. The suppliers cannot charge higher prices and sell the products directly to the consumers and that leads to the provision of high quality products because of the evidence that most products are the generic versions of the patented products that satisfy 80% of the market needs while only 20% are produced locally. Most of the products are semi-finished medicaments.
The competitive advantage is based on the premise that the companies cannot shift the operating costs to industry players because of the diversity of companies that provide input products for the manufacturing of pharmaceutical products, which represent a significantly high volume of business in the industry.
The result show that the companies that supply the raw materials lack the bargaining power because the relative size the suppliers is big and diverse, the number of competing brands is small, distribution channels and supply chain systems for the ten dominant players have already been established, and the rate of growth and expansion of the industry is fast leading to increased competition and lower revenue and attractiveness of the industry.
However, the facilitating factor for the observed growth is the strong economy of Saudi Arabia. Despite the products being unique, it is evident that more companies could be interested to enter the industry because of the high profits and dynamically growing market, a fact that is likely to increase the rivalry from competitors. However, the switching costs from one substitute product to another are high and that makes it difficult for companies to switch and be effective in market. In theory, companies switch to new and differentiated products in the pharmaceutical industry because of the large profits that are made by companies that provide patented drugs.
Here, it is not possible for new companies to enter and sell directly into the markets because of the high cost of setting up the required infrastructure and the competition from those companies already selling directly into the market. However, it is possible to attract new entrants into the market if those firms operating in the industry make large profits. However, the double-digit economy is an incentive for new entrants, a fact that companies must consider when defining new competitive strategies based on Porter’s five forces.
Most companies have adopted a strategy of competitive collaboration by establishing partnerships to reshape competition in the favour of the players and to shape the supplier side economies of scale so that the fixed and variable costs can be distributed among the companies according to specialisation.
The results show that the power of buyers is a drive of profitability and the size of the market in Saudi Arabia is the driving force for competition. Buyers create a network effect depending on the needs that drive them. Here, branding, pricing, patented drugs, prescriptions, and the effects of generic drugs are factors that a company must consider when formulating a competitive strategy. Typically, statistical data provide empirical evidence that the Saudi Arabia Market is wide and growing dynamically for pharmaceutical products.
Evidence shows a mean of 3.17 and a standard deviation of.197 are interpreted to mean that the size of the market is large, but competitive advantage is moderate among the companies in the industry. However, capital requirements could underpin the competiveness of any company because the size of the market could require large capital investments such as extending customer credits, which in turn is risky for small operators because it is a barrier for expansion.
Here, unrecoverable capital could make it difficult for smaller companies to finance expenditure despite the moderate level (standard deviation of.963) of competitive advantage. It is important to note that the capital requirements and financial statements may deter smaller companies from shaping the growth strategy and expand quickly into the rapidly expanding market.
The threat of new entrants into the industry is an additional source of forces that shape competition and strategy. It is possible for the profits enjoyed by companies in the market to fall rapidly if barriers are not erected to deter new entrants from joining the market. However, the booming economy of the government of Saudi Arabia supports the trend in which new companies have been making entries into the industry at a 40% rate. The observed trend is in agreement with the observed responses (M=3.13) where consumers prefer locally manufactured product to the imported pharmaceutical products, the competitive pricing strategies (M=3.17), and high level of difficult in switching to new products (M=2.46), a strong local economy, and the moderate advantage of investors in the industry. It is impeccable to conclude that
However, the moderate effects are based on the industry structure that is relatively stable with persistent profits, a wide market, loyal customer base, and a strong economy (the largest in the Gulf region with a double digit growth). Here the factors constitute the desire by new companies to enter the market despite the legal environment is Saudi Arabia operating under the nation Health Laws and provisions by the Medicines Regulatory Authority that regulates the mandatory requirements for importing medicine and related products into Saudi Arabia.
The supporting static shows a mean of 3.17 for loyalty that leads to a moderating effect on competitive advantage. Here, the statistics of the investors willing to establish new companies show a mean of 2.38), which supports the dynamically increasing number of local companies, government regulations that deter investment in the industry (M=2.42), highly differentiated products (M=3.17), and moderate startup costs (M=2.75), and new firms fearing competition to set up new companies (M=2.38) being unfavourable. Statistical and analytical evidence show that barriers to entry are moderate. Substitute products have a wide market base (M=3.13) and a moderate effect with a p-value of.524, which shows the effects could be strong if manufactured locally.
Theoretical
The Porter’s five forces model provides the foundation for shaping the competitive strategy among the 271 national and international companies operating in the pharmaceutical industry in Saudi Arabia. The model identifies the vertical and horizontal forces of competition in the pharmaceutical industry in Saudi Arabia (Maignan & Lukas 1997). The buyer and suppliers define the vertical component while the horizontal component is described by the top 10 rivals, entrants wanting to join the market because of the double digit economy of Saudi Arabia, and substitute products that show a moderate effect on the competitive advantage of the firms.
Here, the root cause of profitability in the industry and the underpinnings of competition leads to the conclusion that local and international companies operating or intending to enter the pharmaceutical market should establish how best to shape profits both in the branded and generic products by tying Porter’s Five Forces model to designing the competitive strategy.
Methodological
The research methods and tools for data collection and analysis show methodological reliability because of the compliance with Porter’s five forces model. However, the methodological success of the study shows the link between strategy formulation and how managers can leverage competitive information for competitive advantage. However, the downside of the study is that it does not show the regulatory framework for companies applying to establish local companies to manufacture pharmaceutical products. Based on Porters five forces model, it is evident that it is not possible to determine the competitive interactions among the firms, which made it difficult for the researcher to focus the investigation on individual firms.
Practical issues
In practise, the top ten firms have a competitive edge when evaluated against the 270 firms in the pharmaceutical industry in Saudi Arabia. The companies have already established strong links with the suppliers and the market with strong brand names that could allow them to fight off the threats from substitute products and new entrants. New entrants and brands could find it difficult to enter the market despite the findings which show only moderate effects of the competitive forces. The rationale is that branded companies will employ all legal channels and large financial resources to create secondary patents on existing products so that manufacturers of generic products cannot compete in the same market and space.
The strategy will compel interested firms to invest in research and development projects so that the playing ground becomes level from the cost, research, and development perspectives. It is possible for branded firms to impose costs on interested firms, which could deter new firms from entering the market with generic products that branded firms have invested heavily to develop. It is evident that the strategy works well for branded firms in fighting off competition, make smaller firms to consider their profit margins to sustain and increase the market and profit margins. However, the new firms can acquire inputs and other key ingredients at premium prices. Despite that, the government of Saudi Arabia can provide support for local firms to access key ingredients to enable cheaper production of generic products.
Recommendations
Companies with branded names and strong market sharers and performance evidently enjoy competitive advantage over rival firms that are mere starters in the industry. However, home-grown solutions in the pharmaceutical industry in Saudi Arabia is critical because of the size of the industry, creation of jobs, and establishment of companies that product quality products based in research and development programs, which the country can invest in to boot local manufacturer. Here, the government and the citizen are bound to benefit radically if the government supports the establishment of local firms and the production go generic products besides investing in research and development.
Questionnaires
References
Ahuja, S & Scypinski, S 2010, Handbook of modern pharmaceutical analysis, Academic press, New York.
Alakloby, O M 2005, ‘Pattern of skin diseases in Eastern Saudi Arabia’, Saudi medical journal, vol. 10, no.26, pp. 1607-1610.
Al-Wazaify, M, Matowe, L, Albsoul-Younes, A & Al-Omran, O A 2006, ‘Pharmacy education in Jordan, Saudi Arabia, and Kuwait. American journal of pharmaceutical education’, vol. 1, no. 70. pp. 10-12
Asiri, Y A 2011, ‘Emerging frontiers of pharmacy education in Saudi Arabia: the metamorphosis in the last fifty years’, Saudi Pharmaceutical Journal, vol. 1, no. 19, pp. 1-8.
Bingham, C B & Eisenhardt, K M 2008, ‘Position, leverage and opportunity: a typology of strategic logics linking resources with competitive advantage’, Managerial and Decision Economics, vol. 2-3, no. 19, pp. 241-256.
Braithwaite, J 2013, Corporate Crime in the Pharmaceutical Industry (Routledge Revivals). Routledge, New York.
Conklin, D W 2010, The global environment of business: New paradigms for international management, Sage, New York.
Eljelly, A M 2004 ‘Liquidity-profitability tradeoff: an empirical investigation in an emerging market’, International Journal of Commerce and Management, vol. 2, no. 14, pp. 48-61.
Enright, M J 2003, ‘Regional clusters: what we know and what we should know’, Innovation clusters and interregional competition, vol. 1, no. 1, pp. 99-129.
Fina, E & Rugman, A M 1996, ‘A test of internalization theory and internationalization theory: The Upjohn company’, MIR: Management International Review, vol. 1, no. 1, pp. 199-213.
Frank, R G 2007, ‘The ongoing regulation of generic drugs’, New England Journal of Medicine, vol. 20, no. 357, pp. 1993-1996.
Fung, M, Thornton, A, Mybeck, K, Wu, J H H, Hornbuckle, K & Muniz, E 2001, ‘Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets-1960 to 1999’, Drug Information Journal, vol. 1, no. 35, pp. 293-317.
Gill, J & Johnson, P 2010, Research methods for managers, Sage, London.
Harrigan, K R 1984, Joint ventures and competitive strategy, Sage Publication, London.
Ingelman-Sundberg, M, Oscarson, M & McLellan, R A 1999, ‘Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment’, Trends in Pharmacological Sciences, vol. 8, no. 20, pp. 342-349.
Kheir, N, Zaidan, M, Younes, H, El Hajj, M, Wilbur, K & Jewesson, P J 2008, ‘Pharmacy education and practice in 13 Middle Eastern countries’, American journal of pharmaceutical education, vol. 6, no. 72, pp. 10-27.
Kotler, P & Armstrong, G 2010, Principles of marketing, Pearson Education, London.
Kulkarni, M G 2004, ‘Foresight as a source of competitive advantage in the global polyolefin industry’, International Journal of Foresight and Innovation Policy, vol. 1, no. 3-4, pp. 27.
Lazar, J, Feng, J H & Hochheiser, H 2010, Research methods in human-computer interaction, John Wiley & Sons, New York.
Lee, Z A, Ahmad, Z, Ahmed, I & Nawaz, M M 2010, ‘Organizational climate (OC) as employees’ satisfier: Empirical evidence from pharmaceutical sector’, International Journal of Business and Management, vol. 10, no. 5, pp. 214.
Maignan, I & Lukas, B A 1997, ‘Entry mode decisions: the role of managers’ mental models’, Journal of Global Marketing, vol. 4, no. 10, pp. 7-22.
Paul, S M, Mytelka, D S, Dunwiddie, C T, Persinger, C C, Munos, B H, Lindborg, S R, & Schacht, A L 2010, ‘How to improve R&D productivity: the pharmaceutical industry’s grand challenge’, Nature reviews Drug discovery, vol. 3, no. 9, pp. 203-214.