Abstract
This study focuses on issues surrounding the diagnosis and management of patients with Chronic Obstructive Pulmonary Disease (COPD). The paper examines a COPD patient called Mr. D.H. According to an article by Total Nutrition Therapy (n.d), Mr. D.H, who is now 68 years old, has been under COPD medication for several years. Mr. D.H., who is on home oxygen treatment, presents with dyspnea, exertions, and continued frequent exacerbations. Previous episodes by D.H. demonstrate infection in the upper respiratory, which caused increased sputum and difficulties in breathing. This study carries out a diagnostic and management plan for Mr. D.H’s condition. The prevalence of COPD in the United States among males and females also becomes highlighted. Further, the pathophysiology, differential diagnoses, and management plan of the OCPD patient become incorporated into this study.
Assessment
Spirometry assessment demonstrated an FVC prediction of 63%, a FEV1 prediction of 26% and a FEV1/ FVC ratio of 0.5.
Clinical Significance
Mr. D.H’s spirometry results became classified as post-bronchodilator (FEV1 26% predicted). This demonstrated that Mr. D.H was in Stage 4 of COPD, which is an extremely severe stage. In other words, Mr. D.H experienced severity when it came to airflow impediment.
Clinical Presentation
Mr. D.H presents with dyspnea, exertions, and continued frequent exacerbations.
Incidence of COPD in Male, Female and Total Death in the US
COPD is a leading cause of death around the globe, although, it is highly prevalent among smokers (Maclay, Rabinovich, & MacNee, 2009). Many Americans suffer from this condition, and there is a risk of increase in the number of people who become infected. Research demonstrates that COPD, in the United States, affects more women than men suffering from the diseases as demonstrated in the following table (Dyess, 2011).
Diagnostic and Laboratory Testing
- No signs of central cyanosis or discoloration of mucosal membranes
- No swelling of ankles or legs
- Use of scalene muscles for respiration
- Barrel-shaped ribs
- Reduced breath sounds
- Arterial blood gas measurements show a PaO2 of 9.2 kPa
- Chest X-ray shows outstanding bronchial markings
Implication to Practice
Since PaO2 is not less than 8.0kPa, there is no case for respiratory failure. Outstanding bronchial markings that occur in chronic bronchitis appeared after conducting chest x-rays. Hence, there is a risk of significant commodities such as cardiac failure.
Pathophysiology
COPD brings about pathologic changes in the lungs, which trigger physiologic changes such as airflow inadequacy, ciliary dysfunction, pulmonary hyperinflation and hypertension, as well as, too much secretion of mucus (Maclay et al., 2009). The production of excess mucus causes chronic coughs that may be accompanied by sputum production (Blackler, Jones, & Mooney, 2007). Restriction in expiratory airflows occurs due to inflexible, airway impediment and the resulting rise in airway resistance. On the other hand, the impediment of peripheral airways, pulmonary defects and parenchymal devastation, in late COPD phases, may cause hypoxemia (Maclay et al., 2009). In severe COPD, pulmonary hypertension acts as the main source of cardiovascular complications (Blackler et al., 2007).
Regular COPD indicators comprise dyspnea, coughs, and generation of sputum (Blackler et al., 2007). Normally, symptoms of COPD first appear in persons aged above 40 years, and COPD exacerbations appear in persons above 50 years. Geriatric patients usually undergo severe exacerbations, which call for hospitalization. On the other hand, COPD is not common in children, although, children who experience chronic coughs and difficulties in breathing may be examined for asthma and cystic fibrosis. Also, persons who have a history of exposure to risk factors linked to the illness need to be diagnosed (Lundback, Lindberg, & Lindstrom, 2008).
Spirometric measurements for Mr. D.H were taken. Spirometry may diagnose COPD by measuring expiratory airflow limitation as shown in figure 1.
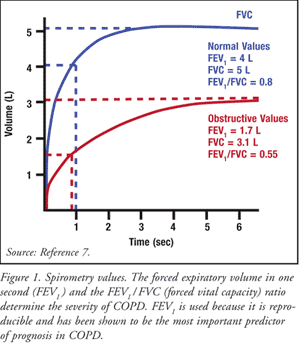
Spirometry entails measurements of Forced Vital Capacity (FVC) and Forced Expiratory Volume in one second (FEV1) and calculation of FEV1/ FVC ratio (Blackler et al., 2007). Spirometric outcomes should be indicated as % projected using suitable normal values for an individual’s age, height and sex.
Mr. D.H’s spirometry results became classified as post-bronchodilator (FEV1 26% Predicted, which demonstrated an extremely severe case of COPD.
Stages of COPD depend on the severity of airflow impediment. These stages range from mild COPD to severe COPD. All stages have an FEV1/FVC of <7. Stage 1, which is a mild stage, has an FEV1 % predicted ≥ 80%. Stage 2, which is the moderate stage, has an FEV1 % predicted at 50% -79%. Stage 3, which is the severe stage, has an FEV1 % predicted at 30%- 49%. Lastly, Stage 4, which is the extremely severe stage, has an FEV1 % predicted that is < 30% (Blackler et al., 2007).
Both symptoms and spirometric outcomes became considered before constructing an individualized management plan for Fred.
Differential Diagnoses
Mr. D.H went through two differential diagnoses. The first differential diagnosis was bronchiectasis. Bronchiectasis is a key differential diagnosis of COPD. In most cases, it is impractical to distinguish patients with COPD from those with bronchiectasis through regular methods of testing (Blackler et al., 2007). This is because bronchiectasis is characterized by large amounts of sputum production, which could also be a sign of COPD. Chest X-ray was carried out for differentiation. In cases of bronchiectasis, chest X-rays demonstrate thickening of bronchial walls. The other differential diagnosis was tuberculosis. Similar to bronchiectasis and COPD, tuberculosis involves sputum production. Hence,the need for differential diagnosis of the ailment. In cases of tuberculosis, X-ray on the chest demonstrates nodular lesions or lung permeation.
COPD Management Plan
These are the main goals of COPD management.
- To alleviate symptoms.
- Stop the progression of the illness.
- Treat and prevent complications and exacerbations.
- Lessen mortality rates.
Algorithm 1: Diagnosing COPD
Mr. D.H was aged 68 and, although, he did not have a history of smoking, he exhibited dyspnea, exertions and continued frequent exacerbations (Total Nutrition Therapy (n.d).
Ahead of diagnosis, Mr. D.H answered questions regarding the presence of factors such as chest pains, fatigue, weight loss, coughs and occupational hazards. Mr. D.H reported cases of fatigue and weight loss. A Spirometry was then performed for assessment of the level of FVC and FEV1, to establish the level of COPD in Mr. D.H (Currie, 2009).
Algorithm 2: Reducing Risk Factors
Mr. D.H’s records did not demonstrate any history of smoking. However, most of the worlds have many smokers and Mr. D.H’s environment could not have been any exceptional. Hence, it was crucial to enlighten Mr. D.H regarding the dangers caused by smoking, and how to ensure that he does not get influenced into smoking. Non-pharmacological strategies such as counseling became employed in order to see that Mr. D.H does not turn into smoking (Talwar, Jain, & Vijayan, 2006). He was also introduced to programs on smoking cessation, which are key to controlling smoking (Talwar et al., 2006).
Follow-ups
Mr. D.H’s case requires many follow-ups as it is in the last stage. Patients in severe stages require many follow-ups compared to patients in the initial stages of COPD, who do not require regular reviews. Clinical assessments of Mr. D.H should include FVC, FEV1, body mass index, and dyspnea scores. Other aspects of Mr. D.H’s clinical assessments should include control of symptoms, treatment of complications, assessment of impacts caused by drug treatment, as well as, examination for any referral or rehabilitation needs.
Referrals
Mr. D.H became referred to other departments because of the low level of FEV1, presence of severe OCPD and the need to evaluate his lung volume reduction surgery. Mr. D.H did not see a respiratory physician, as his case could be handled by COPD team members who had suitable training and proficiency in the field.
Algorithm 3: Manage a well established COPD
Mr. D.H obtained further treatment since his illness had increased. He obtained both pharmacologic and non-pharmacologic treatment. Managing a well-established COPD entails a progressive increase in treatment, in line with the severity of illness. Pharmacologic treatment both lessens the severity of exacerbations and prevents symptoms associated with COPD (Currie, 2009). Mr. D.H obtained additional drugs including theophylline, inhaled bronchodilators and glucocorticosteroids. These are the main treatments using bronchodilators (Blackler et al., 2007). The selection of the drugs relies on the availability of the drug and the reaction of patients. Treatment using bronchodilators helps in managing COPD symptoms. Bronchodilators should only be administered on a regular basis, or when need arises in order to lessen or prevent symptoms. While bronchodilators have side effects, they lack adverse effects (Diaz, Bruns, Ezzie, Marchetti, & Thomashow, 2008). Mr. D.H also obtained training on how to use inhaled bronchodilators.
Bronchodilators of all types enhance exercise capability in COPD, without essentially creating noteworthy alterations in FEV1 (Diaz et al., 2008). Use of inhaled glucocorticosteroids for regular treatment should be administered for symptomatic patients who have FEV1 < 50% predicted (Falk, Minai, & Mosenifar, 2008). Patients with recurring exacerbations should be treated with oral glucocorticosteroids or antibiotics. Systemic glucocorticosteroids could not be administered, due to an adverse benefit, to risk ratio (Falk et al., 2008). Education was also offered to Mr. D.H. Education is useful in enhancing health status of COPD patients (Blackler et al., 2007). Education is also efficient in realizing some goals such as smoking cessation (Currie, 2009). Besides, patient education is efficient while starting discussions on advanced directions and end-of-life issues, as well as, enhancing patient reactions to acute exacerbations. Some settings may be appropriate for education including pulmonary rehabilitation programs, home care programs and outreach programs. Education must be customized to the culture and needs of the patient. Such education should be practical and interactive (Currie, 2009). Some areas that became covered in the education program included crucial information regarding COPD, self-management, therapy and features of medical treatment, guidance on when to ask for help, managing exacerbations and smoking cessation.
Also, Mr. D.H received pulmonary rehabilitation. Pulmonary rehabilitation should enhance life quality, decrease symptoms and boost emotional and physical involvement in daily activities. Therefore, pulmonary rehabilitation covered many nonpulmonary issues such as social isolation, exercise, loss of weight and depression to achieve these aims. COPD patients in all phases of illness gain from exercise training arrangements (Falk et al., 2008).
Algorithm 4: Managing Exacerbations
Mr. D.H’s exacerbations became handled through home care with medical interventions (Falk et al., 2008). This is because Mr. D.H experienced frequent exacerbations. Hence, the suggestion for referral and management of COPD exacerbations came from both the hospital management and Fred (Blackler et al., 2007). Mr. D.H experienced acute exacerbations, which could be managed from home with treatments like theophylline, inhaled bronchodilators and glucocorticosteroids (Blackler et al., 2007). Antibiotics were also administered to Mr. D.H, since Mr. D.H experienced dyspnea, cough and increased amount of sputum (Currie, 2009).
In conclusion, Mr. D.H’s spirometry results can be classified as post-bronchodilator (FEV1 26% Predicted). Mr. D.H’S FEV1/FVC and FEV1 are extremely low, implying that his case is severe.
Mr. D.H’s exacerbations require medical interventions. This is because Mr. D.H experiences both dyspnea and frequent exacerbations. Efforts to provide home care to Mr. D.H have not been extremely successful, as his condition has deteriorated over the years. Lastly, efforts to see that Mr. D.H does not start smoking must be put in place. This is because Mr. D.H’s environment is filled with smokers, who may influence him to start smoking. Non-pharmacological strategies such as counseling must be employed to see that Mr. D.H does not get trapped in the habit of smoking. Also, Mr. D.H’s case requires many follow-ups as it is in the last stage.
References
Blackler, L., Jones, C., & Mooney, C. (2007). Managing chronic obstructive pulmonary disease. Chichester, England: John Wiley & Sons.
Currie, G. (2009). Chronic obstructive pulmonary disease. New York, NY: Oxford University Press.
Diaz, P.T., Bruns, A.S., Ezzie, M.E., Marchetti, N., & Thomashow, B.M. (2008). Optimizing bronchodilator therapy in emphysema. Proceedings of the American Thoracic Society, 5(4), 501-505.
Dyess, D. (2011). U.S. rates of COPD stabilize while women show higher occurrence. Web.
Falk, J.A., Minai, O.A., & Mosenifar, Z. (2008). Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society, 5(4), 506-512.
Lundback, B., Lindberg, A., Lindstrom, M. (2008). Not 15 but 50% of smokers develop COPD: Report from the obstructive lung disease in Northern Sweden studies. Respiratory Medicine, 97(2), 115-122.
Maclay, J.D., Rabinovich, R.A., & MacNee, W. (2009). Update in chronic obstructive pulmonary disease 2008. American Journal of Respiratory and Critical Care Medicine, 179(7), 533-541.
Talwar, A., Jain, M., & Vijayan, V.K. (2006). Pharmacotherapy of tobacco dependence. Medical Clinics of North America, 88(6), 1528-1529.
Total Nutrition Therapy (n.d). Case study 3: Chronic Obstructive Pulmonary Disease (COPD). Total Nutrition Therapy, 2, 173-181.