Introduction
Opiate abuse has grown over the years, currently reaching crisis levels. One in eleven Americans could be classified as either dependent on or an abuser of illicit drugs as described in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSMIV, 2008) guidelines. A significantly larger population, as estimated by the Substance Abuse & Mental Health Services Administration (2009a) revealed that close to 3.9 million individuals are habitual illicit drug abusers. A nationwide study carried out in 2008 by National Survey on Drug Use and Health (NSDUH) revealed that the incidence of illicit drug use was at 8%, in the teenage and adult population (or 20.1 million people) with 4 % subscribing to the usage of a hard. Of the commonly used illicit drugs, marijuana was the most popular followed by cocaine, hallucinogens (e.g. Ecstasy). Prescription psychotherapeutic drugs ranked last in the group. The incidence of experimentation, recreational use and outright addiction to the first three drug classes has remained the same from the 2007 survey findings (Substance Abuse and Mental Health Services Administration, 2009a).
The magnitude of the situation is bordering on astronomical levels. However, even with these levels, approximately 2,300 out of 13,688 substance abuse treatment facilities, including hospital-attached, stand-alone inpatient and outpatient services in the United States have been declared redundant (Substance Abuse and Mental Health Services Administration, 2009b).
Detoxification Programs
Prior to rehabilitation, patients must go through a process of drug detoxification, a procedure that many times seems like an ordeal. During the procedure the uptake of the addictive substances is usually controlled for withdrawal to be attained. At the same time purging of the by-products or remnants of habitual consumption occurs. This process takes an appreciable amount of time and more often than not is excruciating. The detoxification procedure, therefore, requires a collection of interventions aimed at mitigating pain and other symptoms. According to the United States Department of Health and Human Services (2006), there are three principal detoxification stages. These are:
Assessment and Evaluation: On arrival at the detoxification establishment, the patient is taken through a battery of tests targeted at determining the psychological effects of the abused substance to the user. Behavioural issues that precipitate or aggravate the substance abuse, and fortitude for undergoing the detoxification processes are carefully analyzed during this stage. The assessment also includes physical examination for morbid conditions (such as liver function tests in the case of alcohol abusers) in order to determine the amount of organ (liver) damage caused by the drug.
Stabilization or actual detoxification: The first step of the process is admission of the patient. This stage begins with the patient being briefed on what to expect, the anticipated period for the detoxification procedure, and events associated with post-withdrawal recovery. Once the patient has fully understood the process, clinicians start the procedure. In the initial stages, the patient may go through physical trauma so this stage of the process may employ the use of drugs to help mitigate the suffering. The presence of family members and other social support is crucial at this stage of rehabilitation (Office of Applied Studies, SAMHSA, 2010).
Transition to Rehabilitation or Other Treatment: As soon as the detoxification centre or department has helped the patient through the experience of resolving physical dependence and physiological addiction, the process of counselling is initiated to help address psychological dependence. This is reinforced by encouraging the patient to go through a rehabilitation program. The patient is then moved to either an inpatient or a residential rehabilitation facility (U.S Department of Health and Human Services, 2006).
Literature Review
Opiate dependence remains one of the fundamental challenges that continue to plague the healthcare system thereby making it a major social problem in the United States. Opiate dependency has resulted in an upsurge in blood borne disease such as HIV/AIDS, and tuberculosis and led to sky rocketing medical costs thereby affecting the overall prioritization of policy initiatives at state and national levels. This has underscored the need for the development of treatment strategies aimed at treating as many people as possible. The safety and the policy issues related to the utilization of the treatment options need to be put in place. Evaluation and recording of the results of the various treatment regimes is paramount to ascertain their effectiveness and suitability to the society (Bridge, Fudala, Herbert & Leiderman, 2003).
Medication-Assisted Treatment
Addiction is a chronic disease that can be effectively treated. However, due to the nature of the disease, it has a profound effect on the central nervous system and the general psychosocial system as well. It is very easy for opiate abusers to relapse if the previous conditions that led to the dependence are still present. The trends in relapse rates are comparable to the ones observed in other chronic diseases with both behavioural and psychological components such as diabetes and asthma.
The occurrence of the relapse may indicate a failure of the treatment regimen provided. However, various scholars have fronted the notion that relapse is not a sign of failure but rather an indicator that the treatment regimen being used is not suitable for that particular patient.
Medication assisted treatment is a pharmacotherapy approach involving the combination of counselling and pharmacological strategies in the treatment of alcohol and drug abuse. Administration mainly helps address the issues that accompany withdrawal such as anxiety, vomiting and nausea. Because of the different presentations of the various addictive drugs, each addiction requires specific medication/s. This part of the essay will review the most common medications used in the treatment of opiate addiction.
Buprenorphine (Suboxone, Subutex)
Applied Pharmacology: Studies conducted recently by the National Institute on Drug Abuse (NIDA, 2008) concluded that buprenorphine has both agonist and antagonist characteristics. Being a partial agonist and an opioid, it is associated with both positive and negative opioid effects. The negative effects include compromise in the respiratory system and euphoria. However, its combined opioid effects are much less that those produced by full opioids such as heroin and the prescription drug methadone (Center for Substance Abuse Prevention/SAMHSA, 2009).
When administered at low doses, Buprenorphine is capable of eliciting sufficient agonistic effects to make an individual withdraw from opioid dependence without necessarily experiencing the withdrawal symptoms. The agonist effect increases with the increase in the dosage of the drug and continues until such a point as a plateau is attained (Robotham, 2005). At this saturation point, the net agonist effect stops increasing even when the dosage is increased further. This is generally referred to as the ceiling effect. The ceiling effect, therefore, makes buprenorphine have minimal risk for dependence and abuse, or for side effects, in comparison to full opioid agonists (Gowing, Ali, & White, 2001). In some instances, buprenorphine can halt the effects of full opioid agonists and may result in a patient eliciting symptoms of withdrawal. This is particularly true if the individual suffers from acute intoxication at the time of detoxification.
Administration method: Buprenorphine can be administered through intravenous, intramuscular or sublingual routes.
Intravenous: Buprenorphine is easily distributed within body tissues and this helps it acquire a substantial distribution volume (10 L/ Kg). The distribution is divided into two phases: – a rapid distribution stage and a slow elimination phase. The rapid distribution phase takes an average of two minutes while the elimination phase has a half-life of between three and five minutes (Ducharme & Luckey, 2000). A further slow elimination phase greater than 24 hours has occurred. The clearance of the drug from the body occurs mainly via biliary excretion. Some traces of the drug and its metabolites are however noticeable in the kidney. The drug and its metabolites go through the enterohepatic system and this, accompanied by the slowness of excretion, explains the extensive terminal elimination phase. The IV administration of has a shorter bioavailability due to rapid absorption and elimination.
Intramuscular: When administered via the intramuscular route, buprenorphine is rapidly absorbed in five to fifteen minutes. The bioavailability (40-90%) is also desirable compared to the intravenous route. As when administered through the intravenous route, buprenorphine delivered intramuscularly is eliminated in two phases (Robotham, 2005).
Sublingual: When buprenorphine is administered via the sublingual route, the rate of absorption is generally low, with a mean of about 200 minutes. The bioavailability of the drug when administered via this route ranges between 16 and 94 percent (an average of 55%). The elimination profile for the sublingual route is the same as for the intravenous and intramuscular route, presenting with the two elimination phases (Mattick et al., 2002). Plasma half-lives are three to five hours for the first phase and more than 24 hours for the second phase.
Formulation: Like all other forms of addiction an oral dosage of buprenorphine is preferred for opioid addictions. The sublingual route offers a better bioavailability than other routes.
When used to produce analgesic effects, it is more effective than morphine primarily because morphine has a high tendency for addiction (Conrad, & Schneider, 1992). A 0.4 – 0.6 mg dose of sublingual buprenorphine is equivalent to 10mg of morphine administered via the intramuscular route.
Safety: The ceiling/plateau effect associated with buprenorphine, coupled with good bioavailability, makes the drug very safe to use. This is particularly true if used in overdose. And compared to full opioid agonist, the side effects associated with buprenorphine are easy to manage. Generally, maximal effects of the drug present in a dosage range of 16 mg to 32 mg when administered via the sublingual route (Mattick et al., 2002). If doses surpass this range, then the effects are likely to increase as well.
Incidents of respiratory depression associated with a buprenorphine overdose have a less likelihood than if other opioids such as Methadone are utilized (Gowing, Ali, & White, 2000). There have been no reports of liver damage associated with an overuse of buprenorphine. However, various studies have shown that some slight enzyme increase occurs within the liver if the drug is used chronically. The drug has not been associated with significant interruption of cognitive and psychomotor functions.
Though there is limited availability of statistical data on the use of buprenorphine in pregnancy, very few significant complications have been reported and both suboxone and sabutex have been regarded by the Food and Drug Administration (FDA) as belonging to the Category C of pregnancy medications.
Abuse Potential: According to the literature, individuals with limited access to opiods have been identified as most at risk (Conrad, & Schneider, 1992). More often than not, naloxone is used in addition to buprenorphine to reduce the chance of dependency on the latter. When administration of both drugs is given in a tablet form, the opioid agonist consequence of buprenorphine dominates and naloxone does not initiate withdrawal. Alternatively, the tablets can be ground and administered through injection, however this may result in elevated levels and severe withdrawal symptoms.
In some instances, buprenorphine can bring about an opioid syndrome on its own. This happens when there are high levels of dependence, particularly physically. The same can also occur if the interval between the dosage of the opioid agonist (such as methadone) and a dose of buprenorphine is less than two hours (Ducharme & Luckey, 2000). High doses of buprenorphine can also bring about symptoms associated with withdrawal.
Evidence of Effectiveness: Recent studies have demonstrated that the efficacy of buprenorphine is much higher than that of a placebo. An analysis of trials by Mattick et al. (2002) revealed that buprenorphine, when administered at moderate (8 mg- 15mg) doses and at higher (16 mg) doses, can result in a reduction in the use of heroin. The efficacy of buprenorphine compared to placebo was found to be significantly higher. In 24 studies involving 4497 individuals, it was found that the retention rate of buprenorphine was superior to placebo (Mattick et al. 2002). For low dosages the retention rate was 1.50 which was a 95% average while the concealment was between 1.19 – 1.88. For medium dosages the retention rate was 1.74; a 95% average with a concealment of 1.06 – 2.87. For higher dosages, the retention rate was 1.74 as well and the concealment was between1.02 – 2.96. It was, however, realized that only medium and high doses of buprenorphine were effective in treating opioid addiction to levels greater than those attained by a placebo. It has also been found that buprenorphine has the same positive results as an average dosage of methadone in the treatment of opioid dependence. However, buprenorphine is less likely to have the same efficacy rates as an optimal dosage of methadone and this therefore makes it not the drug of choice especially for individuals who present with high levels of dependence.
A study by Gowing, Ali and White (2008) revealed that buprenorphine had some slight advantages over methadone in the treatment of opioid withdrawal syndrome. In particular, their findings indicated that the symptoms of withdrawal resolved much earlier with buprenorphine than with Methadone. The study by Gowing, Ali and White involving 22 trials also revealed that the drug was also more effective than clonidine and lofexidine in the reduction of signs and symptoms that present with withdrawal from opioid dependence (2008). There is no clear evidence that using buprenorphine will ensure that patients entirely withdraw from using opioids. Scholars have concluded that the usage of buprenorphine along with opioids reduces its efficacy (Gowing, Ali, & White 2008). The drug has, however, been championed for having low withdrawal syndrome as compared to other opioids in its class such as methadone. It is therefore favoured as a popular choice in the treatment of opioid withdrawal (Center for Substance Abuse Prevention/SAMHSA, 2009). Buprenorphine therapy takes place in three phases. These include induction, stabilization and maintenance and are summarized below (Krampe, Stawicki & Wagner, 2006).
Induction: This is the point where buprenorphine therapy is begun. The treatment is begun after the opioid addict has rested for a period of between 12 and 24 hours. If the patient has not abstained from opioids for the number of hours specified above, there may be other opioids in his/her bloodstream and this, when combined with buprenorphine, could result in acute withdrawal syndrome (Center for Substance Abuse Prevention/SAMHSA, 2009). The induction phase is normally carried out using suboxone or subutex depending on the outcome of the pre-treatment assessment Stabilisation– This stage begins when the patient has not used opioids for a substantial time and is no longer craving them. During this stage the patient presents with very few, if any, of the side-effects associated with withdrawal (Center for Substance Abuse Prevention/SAMHSA, 2009).
Maintenance: During this stage, the patient is maintained on a stable dose of buprenorphine. The maintenance period varies from individual to individual. Once stabilization is attained the patient can choose not to go through the maintenance phase and instead go into medically supervised withdrawal.
Benzodiazepines
Benzodiazepines are the most preferred medical intervention for opiate withdrawal syndrome. This treatment is administered on an outpatient basis for individuals whose withdrawal symptoms range from mild to moderate. For patients experiencing severe withdrawal, treatment on an inpatient basis is highly indicated. Different formulations of benzodiazepines have proven effective for use in opioid withdrawal. These include chlordiazepoxide, oxazepam, diazepam, alprazolam, lorazepam and halazepam.
The effectiveness of benzodiazepines in the long-term management of opiate addiction has been the subject of contention in recent times. Most researchers focusing on opioid treatment have concluded that the use of benzodiazepines is not be indicated for long-term use. However, from a clinical perspective, many of the individuals suffering from opiate addiction are treated using benzodiazepines during the detoxification stage and later may continue to use them as a treatment for issues like insomnia and anxiety disorders, all related to alcohol dependence (Ries et al., 2009).
Treatment of the symptoms that occur with opiate withdrawal syndrome plays a key role in rehabilitation for patients initially dependent on opium. The main objective is the prevention of patient suffering and reducing the incidence of patient complications such as seizures. Addressing the issues properly greatly increases the success rate of long-term rehabilitation.
The ideal drug for treatment of opiate addiction is one with a rapid onset and a long duration of action. The drug should also have minimal, if any, dependence on liver metabolism and should bear very little potential for addiction. Various benzodiazepines come with both these advantages.
Benzodiazepines offer very effective solutions in addressing a number of issues related to withdrawal. These include help in the control of agitation and the management of seizures associated with withdrawal, prevention of delirium tremens, and cross-tolerant agents associated with alcohol dependence. In addition, benzodiazepines have a diminished ability to develop dependence and come with a reduced potential to develop tolerance during short-course administration. This increases their efficacy in the management of opiate-withdrawal syndrome as the drugs of choice. The widespread use of benzodiazepines has tremendously reduced the incidence of life-threatening issues related to opiate withdrawal such as delirium tremens (Lawrence, 2010).
The use of benzodiazepines in the treatment of opiate withdrawal syndrome has been a subject of much debate. Some researchers have argued that mild to moderate incidents of opiate withdrawal syndrome can be resolved with minimal or without medical intervention (Lawrence, 2010). Other scholars believe that if these mild incidences remain untreated, they will eventually compound thereby greatly increasing in severity. According to the latter group, repeated incidence of withdrawal greatly increases the risk for developing delirium tremens and seizures. The use of benzodiazepines in the treatment of severe cases of opiate withdrawal syndrome is however not a subject of contention. A study by Amato et al. (2005) involving 4,309 individuals undergoing 64 independent tests concluded that benzodiazepines are generally more efficient than placebo in the treatment of opiate withdrawal related seizures although its profile against other popular treatments may be variable.
Detoxification programs
The ever-increasing incidence of drug addiction has led to the establishment of detoxification centers which offer different programs. Dependency on drugs laced with opium such as oxycontin and Vicodin has been on a steady rise and is now identified as a national health hazard in the United States. Opiate addiction has continued to cause mental and physical suffering that eventually leads to depression, termination of careers and breakdown of the society. Rapid detox centers in major hospitals have become operationalized to offer sound and effective methods of continuing care that enhance prevention of relapse. The integration of the most current technology with evidence-based practice is imperative in achieving excellent and positive results in terms of safety, cost effectiveness and relapse prevention. Detoxification programs usually take one to seven hours with a recovery period lasting from two weeks to one month depending on the level of addiction and the effects of the opiate on the functioning of vital organs.
The rapid detoxification program has become popular with patients due to its efficacy and convenience. Centers in Chicago and the Dallas are renowned for their high quality services in rapid detoxification programs. The rapid detoxification allows patients to experience an accelerated and clinically sound procedure that ensures safety due to the high calibre of anaesthesiologists involved in the process. The anaesthesia assisted rapid opiate detoxification procedure offers a smooth transition from dependency through a well-structured and supportive schedule of treatment. Moreover, monitoring of the patients to ensure abstinence usually takes a period of one year (Midwest Rapid Opiate Detoxification Specialists, 2007).
The Waismann Method (Lawrence, 2010) offers the best option for high quality services especially for opiate dependency. Its unparalleled safety and standards in pre-screening of patients has ensured that the method has maintained its grasp as the leader in offering treatment for opiate dependency. The extensive knowledge and the efficiency of the tailored programs coupled with the expertise observed in the medical personnel have served to boost the capacity of the institution.
The rapid detoxification starts with the admission of the patient followed by diagnostic evaluations and physical evaluations before the patient is introduced to treatment under sedation. Despite the fact that the process takes only a few hours, the patient lacks physical dependency after successfully undergoing the process (Anaesthesia Assisted Medical Opiate Detoxification, 2010). The buprenorphine-naloxone has been licensed as treatment option for opium dependent people particularly in programs operating outside normal schedules. The short-term detoxification process was performed on 234 individuals whose age averaged 37 years. The results of the study showed high compliance levels with more than seventy percent of participants completing the dosages. (Anaesthesia Assisted Medical Opiate Detoxification, 2010) Integration of the research findings with practice remained the main driver of the trials involving the treatment programs. The health staff who took part in the study had received prior training to ensure that the research was done successfully. According to Leslie et al (2004), the staff who utilized the Waismann Method received special training to ensure safe detoxification practices. Moreover, the induction procedure of the program achieved more rapid progress than was observed in the majority of other studies. The use of higher starting doses (4 times greater than the normal) resulted in high early treatment rates of compliance. Leslie, therefore, asserted that initiation of buprephrophine treatment can occur early in the management of opiate users including those using street heroin, by office based practitioners using buprenorphine-naloxone tablets. These findings have far reaching implications in the ability of the health care community to reach a wide geographic scope of street heroin users.
Leslie (2004) concluded that the transferability of patients to other treatment strategies required only slight adjustments in the approaches.
In another study opioid dependent patients were put through non-randomized inpatient or outpatient community programs. A comparative analysis found that the outpatient setting recorded higher abstinence rates with comparable retention rates to the inpatient setting. The retention rates in the community programs were found to be comparable to the medical assisted types. It is interesting to note, that demographic factors such as gender, employment and severity of the patients played a major role in influencing the outcome of the treatment, but there was no relationship found between demographic factors and treatment outcome.
Methods that rely on sedating have received widespread criticisms due to their limited safety and follow up procedures. However, measures to improve safety have increased recently, especially in the medication procedures such as designing regimens aimed at minimizing contra-indications such as vomiting. Furthermore, investigations involving buprenorphine showed that the occurrence of adverse effects was limited when either tablets or syrup were utilized and withdrawal symptoms managed (Bickel & Amass, 1999). Studies conducted on the treatment of opiate dependency have largely involved sedatives or depressants.
Fast opiate detoxification, especially when combined common anaesthesia, is among the most efficient and safest methods to suppress withdrawal symptoms. This method is very beneficial for patients exhibiting severe withdrawal symptoms in the course of detoxification and minimizes the chances of relapses occurring.
Methadone treatment is generally known to exhibit pronounced withdrawal symptoms in comparison to that of opiate. However, challenges have been observed in generalizing the observations to different populations. Treatment programs must ensure that they achieve opoid detoxification and eventual sustained abstinence. The introduction of an effective and timely short-term intervention approach offers, potentially, incrementalal relief from a debilitating condition while facilitating creation of a patient-professional rapport and alliance. Programs targeting drug abuse treatments need to focus on fostering incremental planning and promotion of realistic improvements, particularly in the management of heroin addicts (Hser et al, 2001).
Examination of about 3000 admissions recorded in a span of 3 years in a mixed gender detoxification facility showed a significant variation in terms of age, drug use unemployment and higher percentages of parenting status. Callaghan and Cunningham (2002) asserted that consumption of opiate especially as the main primary drug of choice predisposed the individual to high chances of social neglect. They also noted that having alcohol as the main drug of choice acted as a high risk factor for readmission to the inpatient facility. Residential instability coupled with single marital status also contributed to the dropout rates. Moreover, unemployment and instances of multiple drug uses in combination with treatment dropout also contributed largely to the level of readmission. Older people above 37 years were less likely to receive readmission to the inpatient facilities due to the fact they are more mature and ready to reform.
Cost effectiveness
Variations in the costs of the therapies have rendered some treatment to be preffered over others. The National Health Service conducted an assessment to ascertain the cost effectiveness of buprenorphine and methadone maintenance therapy. A comparative analysis of the two treatment regimens showed differences in costs that often tend to influence their utilization in clinical situations. To determine the facyors that led to the differences in the costs Connock et al (2007) picked data from more than thirty systematic reviews and several random controlled trials with the aim of assessing their efficacy in conduction of the treatment in the predominantly youthful category of heroin users. Individualized dosages of the two treatment regimens remained the focus of the studies. Meta-analyses have consistently shown that the Methadone maintenance therapy (MMT) have higher levels of retention, as compared to Bupreprnorphine Maintenance therapy (BMT).
Comparative analysis of the MMT and BMT therapies has consistently been difficult owing to the fact that the modelling employs different approaches, perspectives and different timings. The effectiveness of data obtained as benchmarked against preferred comparison levels influenced the analysis to the extent of invalidating the results. Connock et al (2007) asserted that based on the cost effectiveness different models that are utilized in the United Kingdom such as the flexible dose of MMT and the BMT offers better clinical effectiveness and proves to be more cost effective when compared with the drug free community based therapy. In addition to being more cost effective, the MMT treatment was also found to cause less mortalities and thus scoring high as the preferred detoxification procedure. However, the lack of substantial data on the safety margins of the two regimens is still a major hindrance to the full utilisation of the programs particularly in the United Kingdom.
Studies conducted in California confirmed that MMT was a more cost effective treatment regimen. The cost was benchmarked against the benefits to the patients. The California study noted a benefit –cost ratio of 4.8 when applied in residential treatment that translated to slightly more than $6000 in the first year. Detoxification through sedation has proved the most cost effective compared to the simplest form offered by the self help groups. Drug-free residential treatment was found to be the least cost effective mainly due to the exorbitant residential costs occasioned by the nature of the therapy. Furthermore, legal requirements often stipulate that the therapy lasts for at least three months to ensure that the patients have fully recovered. The residential programs have therefore been found to be less popular as individuals shy away due to the high costs.
Evaluation tools for detoxification program
Evaluation tools have proved imperative in the determination of the treatment outcome especially in the detoxification program. According to Ritcher, Eikelmann & Berger (2004), a short form (SF-36) has proved effective in the monitoring of the health status and in the eventual evaluation of the treatment outcomes in the drug addicts. These findings were obtained from a study conducted in North Western Germany. The evaluation relied mainly on a pre and post assessment of patients who had undergone several detoxification referrals to one of the largest psychiatrist centre in Northwestern Germany. 79 males and 21 females who had a history of abusing different drugs were included in the study. The SF-36 form was provided immediately on admission and was retained until discharge time to ascertain the recorded changes that were largely based on self perceived health status by the patients.
The findings of the study showed that patients who exhibited severe co-morbidity with diseases such as hepatitis B and depression perceived that their health status was poor. Moreover, positive correlations were recorded between the patient self perceptions of their health status and the addiction severity. This was observed by the physicians particularly in cases of mental health status. The study also found that an increased tendency of better results for patients who had been admitted in worse situations (Connock et al, 2007). Other studies have indicated the reinforcement of compliance with treatment regimens and patients’ attendance to post therapeutic gathering.
The Green Spring Health Services (GSHS) Medical Necessity Criteria for Utilisation management has found extensive application in substance abuse control and in particular the determination of the level of care. The GSHS criterion is imperative in the carrying out determinations of the medical necessity particularly for individuals who benefit from services offered by a managed care program (Book et al, 1995). Thus, the needs of patients are realized through concerted efforts. These efforts include the use of complete continuum of chief treatment service options. They range from outpatient detoxification program alone to coupling of the same with partial hospitalization and sometimes an additional supervised residential environment. The American society addiction medicine (ASAM) Patient placement criterion which is purposely oriented towards the treatment planning is often useful in the coordination of patient placement. This usually takes place between the various levels of care as stipulated in the treatment program (Book et al, 1995). The inability to maintain the desired changes after a treatment therapy remain the most serious bottleneck challenging the effectiveness of drug abuse treatment in the society. More importantly, appropriate assessment programs for the techniques used in the treatment programs have failed to ascertain their worth in achieving the laid down objectives. Relapse is generally the failure experienced by a person in the overall maintenance of behaviour change in the long term. Relapse prevention methods are often utilized during primary treatment and in the post treatment interventions. These procedures are particularly designed to enhance patient stability after recovery, especially when they return to their homes.
Relapse prevention strategies tend to fall in the category of cognitive and behavioural therapy models. Moreover, it has been reported that relapse prevention is better done by follow up programs when the patient has fully recovered. Its effectiveness has been recorded in the past, especially in the cases of impaired substance users (Blondell et al, 2008The Community Health Improvement Process (CHIP) model is a tool popularly utilized in the community health planning, which is imperative in assessment of the overall performance of the organization (Issel, 2007). The model has found wide application in monitoring, strategic planning, implementation process and the eventual evaluation of the organization performance. Considering that the study delves on evaluating the efficiency in the program planning in the detoxification and rehabilitation programs, CHIP model forms an appropriate model to help in coming up with the strengths and gaps in the evaluation process (Issel, 2007). The action planning model embarks on the identification and prioritisation of the critical issues in the health sector before analysing them. Development of the indicators is important at this stage since it helps in the monitoring and eventual evaluation of the implementation process (Issel, 2007).
Contributing factors of clients that have returned for detoxification after relapse
Limited evidence exists on the evaluation of predictors, moderators and to a lesser extent the community settings exist. Factors influencing the relapse time include the wearing out that is occasioned by the lifestyle adopted by the patient in a bid to prevent further damage to the functioning of the body (Mann, Charuvastar and Murthy, 1984). The precipitation of life events that is responsible for governing the tendency to distribute the durations taken in each relapse cycle. These factors have major implications in the eventual control of relapses depending on the way they are handled by the by the physicians. To ensure or minimize the occurrence of relapses in the patients, it is usually advisable to apply motivational techniques that enhances or lengthens the period of abstinence among the patients. The kind or type of motivation significantly decides whether the behaviour wills be replicated or the patient will observe the period of abstinence. Mann, Charuvastar and Murthy (1984) observed that motivation and wear out offer contradicting input thereby resulting in the formation of competing risk distribution cycle.
The Nature of Withdrawal
The physiological processes and stresses accompanying withdrawal are similar for both alcohol and illicit drug abuse: symptoms typically include anxiety, muscle aches and pains, insomnia due to disruption of the tranquilizing effects that were associated with the abused substance, diarrhoea and vomiting (Collins et al., 2005). These symptoms involve the parasympathetic nervous system and they manifest whether the patient is awake or asleep.
Non-pharmacological Therapies
In order to address a drug addiction problem, overall attention should be paid to both the individual’s physical and psychosocial functionalities. The use of medication alone has a very low success rate especially in patients with higher levels of opioid dependence (Center for Substance Abuse Prevention/SAMHSA, 2009). Suboxone and Subutex when used as treatment for opioid addiction should be used concurrently with therapies targeted at addressing the behavioural characteristics of the individual (Krampe, Stawicki & Wagner, 2006). Social services need to be effectively provided. Choosing a treatment module for opioid addiction therapy is basically determined based on the dependence levels of the patient. The intensity of addiction treatment ranges from office-based treatment to intensive therapy; the latter demanding the person be given in-patient treatment.
Updates on Group Therapy
Addicts Anonymous is credited with having developed the mutual self-help group counselling approach (Jung, 2009). Group therapy is imperative during recovery in a detoxification centre especially after withdrawal and when stabilization has gained effect. Group therapy has gained recommendation as one of the most important maintenance strategies in rehabilitation centres. In the detoxification facility and subject to evaluation of the particular clinical case, group therapy kicks off the long process of rebuilding self-esteem. This therapy brings about the discipline associated with group dynamics, intensive short-term dynamic psychotherapy, cognitive-behavioural and psychodynamic approaches (Krampe, Stawicki & Wagner, 2006). These aspects assist in strengthening the patients’ will to get off drug addiction and to improve their prospects for success in therapy programs after leaving the detoxification facility.
Mitigating the Pain of Drug Withdrawal with Rapid Detoxification
Some opioid treatment centres advocate and actually do offer medication targeted at rapid or “ultra-rapid” detoxification. Rather than make the patient endure several days of the painful process, a great deterrent for many, these centres compress the core withdrawal procedure into as little as two hours under anaesthesia.
The drugs of choice in rapid detoxification include buprenorphine to clonidine, naloxone, and oral naltrexone or a combination of any of the above. Some positive results have been reported in the various incidences that this regimen has been used. The allure of quickly getting over the agony of withdrawal is offset by pain that continues when the patient awakes from anaesthesia (and quite enough to trigger an 80% dropout rate comparable with non-rapid detoxification methods), severe life-threatening complications and even deaths. On the other hand, Teplin et al. (2005) report that with repeated administration of the Clinical Opiate Withdrawal Scale (COWS), the anaesthesia-assisted rapid detoxification (AAROD) approach results in milder symptoms beginning 24 hours after detoxification ends. This procedure results in detoxification of the body in less than six hours and the patient’s stay in hospital is substantially reduced (Ries et al., 2009).
Nonetheless, the American Society for Addiction Medication (ASAM) cautions that proper clinician training, counselling services and ready access to emergency equipment are vital to the effective practice of rapid detoxification (Collins, Kleber, Whittington & Heitler, 2005). Success rates improve for highly motivated addicts who have the advantage of receiving support from family and friends.
There is heated debate about whether rapid detoxification is ultimately more successful than the slow but reliable maintenance therapy with methadone in the process of opioid substitution. In a review of thirteen treatment trials, the National Drug and Alcohol Research Centre of Australia found that rapid opioid detoxification generally worked to move abusers away from their addiction in a very short amount of time. From the various debates and research conducted, methadone has proved a more effective option in the end, besides costing less over the total course of treatment (Robotham, 2005). Some of the major advantages of methadone include the fact that it prevents cravings for a period of 24 to 36 hours and it halts the mood-altering effects associated with other opioids. Methadone taken evenly for a period of years while under close supervision from a qualified medical practitioner has greater chances of not causing any harm to the patient (Gowing, Ali, & White, 2001).
Benchmarking Program Evaluation
Rationale for and Importance of Evaluation
For the sake of their patients and staff, opioid detoxification centre administrators need to regularly evaluate services and systems with evidence-based feedback in order to establish what works and what needs to be eliminated (Ducharme & Luckey, 2000).. Nearly as vital is the process of establishing regulatory requirements for evaluation of outcomes as the information obtained here will help clients make informed decisions.
Federal regulations and guidelines already make it mandatory for detoxification centres and outpatient facilities to work on improving program performance by continuously measuring patient outcomes. In turn, accreditation bodies approved by the Substance Abuse and Mental Health Services Administration, single-state agencies and managed-care organizations have requested treatment centres to gather objective data on program effectiveness and efficiency [42 CFR, Part 8 § 12(c); Centre for Substance Abuse Treatment, 1999b].
The precise medication assisted therapy (MAT) approach taken by a detoxification clinic and subsequent enrolment in rehabilitation adjuncts appear to be critical variables in outcome assessment (Krampe, Stawicki & Wagner, 2006). According to the American Society of Addiction Medicine (ASAM), less than ten percent of illicit drug and alcohol abusers remain free of their addiction a year later if they undergo detoxification alone. According to Baxter (2007), when the patient completes an appropriate rehabilitation program, the one-year success rate rises markedly to 33% adding four other components to the determinants of detoxification success. These are: After/continuing care (such as being enrolled in a Twelve Step Program), pain management, looking after co-occurring illnesses, and general health care (Baxter, 2007).
Process versus Outcome Evaluation
Given community and regulatory concerns regarding medication-assisted therapy (MAT), in particular benzodiazepine and buprenorphine, the FDA oversight body of Opioid Treatment Programmes (OTPs) has previously emphasized adherence to approved regimens and drug combinations (21 CFR, Part 291). In a review of federally endorsed programs employing buprenorphine, however, the Institute of Medicine (IOM) has in the past helped lead the transition to evaluating patient outcomes. In particular, the IOM has been at the forefront in challenging OTPs to generate direct and valid measures of reduction in opiate and non-opiate drug use, and improvement in positive social function (Cohen, 2008).
Field tests of this initiative to implement outcomes reporting and feedback undertaken as part of the Benzodiazepine Treatment Quality Assurance System (MTQAS) program that ran from 1989 to 1998. While OTP’s were amenable enough to the rationale for performance assessment, it turned out that knowing what action to take on feedback was the barrier to widespread implementation. Largely, the OTP has seemed to lack the technical expertise to identify meaningful program changes or alternatives (Ducharme & Luckey, 2000).
The Benchmark Criteria: Short- and Long-term Effectiveness
Patient progress towards being free of substance dependence is, of course, the prime consideration in any outcome-based evaluation. The immediate goal of detoxification is to help the substance abuser overcome the physiological trauma of withdrawal. After stabilization and recovery, the patient should be able to enter therapy in an opioid-free state. Over the short and medium term, as has been proposed by the American Society of Addiction Medicine (ASAM) benchmarks, the criteria for evaluating effectiveness of procedures include:
(Center for Substance Abuse Treatment, 1999b; Yates, 2005)
Other holistic considerations include vocational skills, consistency of desirable employment, domestic situation, and sociability (Tinkler et al., 2005). These are generally the ideal focus point and rehabilitation programs should provide services targeted at such outcomes.
Substance-free status may be self-reported and supported by physicians providing pain management and general medical care, as well as by responsible personnel at rehabilitation centres being attended. In addition, evaluation of outcomes should be modular particularly along the lines of this contemplated framework:
Project Design
This project will be carried out at the sunrise detoxification centre. It will be a retrospective study where by the data on previous detoxification programs will be analysed. The analysis will include the determination of the effectiveness of the detoxification programs in relation to the adverse effects of the programs and the likelihood of relapses to occur. The project will proceed as follows:
Hypothesis And objectives
The main objective of the project will be to determine effectiveness of the Opiate detoxification programs performed at the sunrise centre. The effectiveness will be determined by examining data on previous detoxification activities to determine the adverse effects associated. The findings will be compared to the existing literature on safety of a detoxification programs for recommendation purposes. The likelihood of the procedure to relapses will also be determined by examining data on patients who reported relapses at the sunrise.
Thus the hypothesis of the study will be:
- Ho: There is a correlation between the patient effects observed and the detoxification procedures used at the sunrise detoxification centre.
- HA : There is no correlation between the patient effects observed and the Opiate detoxification procedures used at the sunrise detoxification centre.
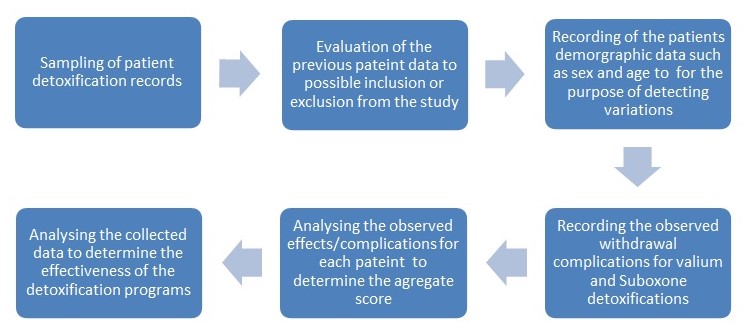
Evaluation of Opiate Dependence Detoxification program at the sunrise
Patient history will be used as the main criteria for inclusion or exclusion from the study. The study aim is to evaluate the effectiveness of the detoxification program and therefore patients with previous complications that may present similar symptoms that may be confused with complications will be excluded. The Specific complications will be underscored against other variables such as age and sex, and previous detoxifications.
Although the study will be a retrospective one, it is necessary to outline the specific procedures involved in the detoxification programs at the sunrise.
Admission for Opiate Detoxification at the sunrise
On admission at the sunrise the patient history is taken by provision of a questionnaire which is completed by the patient and the admitting nurse. The following information is collected; the demographic data, signs and symptoms, the chemical dependency drug used, history of chemical dependency, psychological problems, family interpersonal relationships/living environment, sexuality, previous detoxification if any, Medical problems among others. After the screening the patient is put on an initial detoxification plan with either Valium or suboxone for opiate dependants.
Opiate detoxification admitting orders
The following is done during patient admission for opiate detoxification at sunrise;
- Recording of the patient details; full names, activity and the presence of allergies, this is done by the admitting nurse.
- The vital signs including are checked by medical screening which detects the drugs and other conditions such as pregnancy.
Valium and suboxone detoxification procedures
Both Vaium and suboxone detoxification procedures take between 4 and 14 days for effective treatment to occur. Daily administration of the drugs and monitoring of the patient for complications is performed. The detoxification normally starts on the patient’s second day at the sunrise. The patient’s signs and symptoms during the detoxification are recorded evaluated against the clinical opiate withdrawal scale.
Clinical opiate withdrawal scale
The Opiate withdrawal scale measures the severity of the symptoms associated with the patient withdrawal, details on the opiate withdrawal scale can be found in the Appendix (appendix A)
Randomization and the Sampling Method
Data for total of100 opiate dependents will be randomly selected from the Centre patient files going back 6 months. It is also costs less to do a randomized sample, and despite this, the results obtained are usually an accurate reflection of the situation in the population. All patient records with either the CIWS or COWS forms (or both) filled out will be extracted from the centre database, checked to see which patient and withdrawal medication classification they belong to and listed as part of the project-sampling frame. If the number exceeds the quota of 200, systematic sampling will be carried out to arrive at a final list of patient records. One hundred was chosen as an ideal figure for the project because it would provide for effective data collection and at the same time ensure that the information can be applied in the general detoxification practice.
Ethical Considerations
Approval for this evaluation project will be obtained from the University Ethics Committee. In addition, the evaluator will inform the detoxification centre about the true purpose of the project. Consequently, the evaluator will commit to suppress any information that might lead to unmistakably identifying individual participants. For legal purposes, Informed consent from the patients will be obtained.
Data Analysis
This evaluation will be retrospective in nature. Hence, the population of the study will comprise the patients who completed their detoxification at Sunrise at least two weeks before the project is started. Chi square will be used to test the hypothesis, where by α = 0.05 as the decision rule, meaning a 5% chance of being wrong when rejecting the null hypotheses. If the indicated test shows the probability of occurrence of the observed result due to chance or sampling error at less than 5%, this will mean that the alternative hypothesis will be accepted.
Required Resources
Lead Times
Elapsed Weeks
- Center approval………………………………………………………………………………….. 1
- IRB Approval……………………………………………………………………………………… 3
- Data extraction…………………………………………………………………………………… 8
- Data cleaning, validation, encoding and database set-up………………………… 8
- Data processing………………………………………………………………………………….. 4
- Analysis and Project Report Preparation……………………………………………….. 3
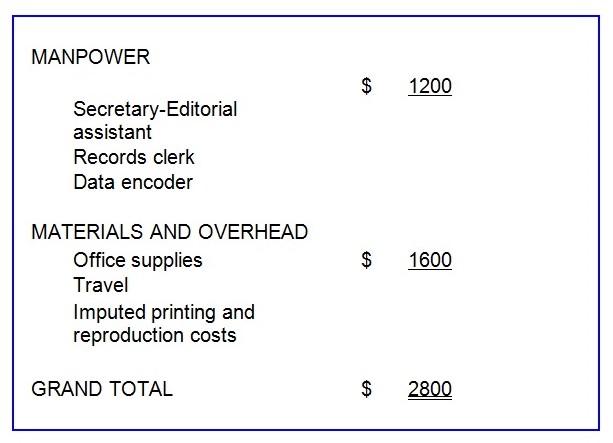
Project Budget
Reference List
Amato, L, Minozzi, S, Vecchi, S, & Davoli, M. (2005). Benzodiazepines for alcohol withdrawal. Cochrane Collaboration.
Anesthesia Assisted Medical Opiate Detoxification. (2010). The Waismann Method sm.
Arnold-Reed, D.E. & Hulse, G.K. (2005). A comparison of rapid (opioid) detoxification with clonidine-assisted detoxification for heroin-dependent persons. Journal of Opioid Management, 1 (1): 17–23.
Baxter, L. E. (2007). Medication assisted addiction treatment: Appropriate use. Princeton (NJ): Division of Addiction Services, New Jersey (NJ) Department of Health and Senior Services.
Book, J., Harbin, H., Marques, C., Silverman, C., Lizanich-Aro, S. & Lazarus, A. (1995). The ASAM and Green Spring Alcohol and Drug Detoxification and Rehabilitation Criteria for Utilization Review. American Journal on Addictions, 4(3): 187-197.
Bridge,T., Fudala, P., Herbert, S. & Leiderman, D. (2003).Safety and health policy considerations related to the use of buprenorphine/naloxone as an office-based treatment for opiate dependence. Drug and Alcohol Dependence, 70(2): S79- S85.
Blondell, R.,Behrens, K., Torsten, B., Smith, Susan, J., Greene, Benjamin, J., Servoss, Timothy, J. (2008). Peer Support During Inpatient Detoxification and Aftercare Outcomes. Addictive Disorders & Their Treatment, 7(2): 77-86.
Collins, E. D., Kleber, H.D., Whittington, R. A., & Heitler, N. E. (2005, August). Anesthesia-assisted vs buprenorphine- or clonidine-assisted heroin detoxification and naltrexone induction: A randomized trial. JAMA, 294 (8): 903–13.
Connock, M., Juarez-Garcia, A., Jowett, S., Frew, E., Liu, Z., Taylor, R.J, et al. (2007).Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technology Assessment, 11(9).
Center for Substance Abuse Prevention/SAMHSA (2009). Medical assisted treatment: Medical community bulletin. Web.
Center for Substance Abuse Treatment (1999b). Guidelines for the Accreditation of Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration.
Cohen, L.M. (2008). Pharmacology and Treatment of Substance Abuse: Evidence and Outcome Based Perspectives. United States: CRC Press.
Ducharme L. J. & Luckey, J. W. (2000). Implementation of the methadone treatment quality assurance system: Findings from the feasibility study. Evaluation & Health Professions, 23 (1): 72–90.
Gowing, L.R., Ali, R.L., White, J.M. (2000) Opioid antagonists and adrenergic agonists for the management of opioid withdrawal, The Cochrane Library, Issue 2. Oxford, Update Software.
Gowing, L.R., Ali, R.L., White, J.M. (2001). Library, Issue 1. Oxford, Update Software. Opioid antagonists under sedation or anaesthesia for opioid withdrawal. The Cochrane Library, Haynes, R.B., Sackett, D.L., Gray, J.M., Cook, Issue 1. Oxford, Update Software.
Gowing, L.R., Ali, R.L., White, J.M. (2008). Buprenorphine for the management of opioid withdrawal. Cochrane Collaboration.
Hser, Y., Polinsky, M., Maglione M. (2001). Matching patients’ needs with drug treatment services. Journal of Substance Abuse Treatment, 16:299–305.
Institute of Medicine (1995). Federal regulation of methadone treatment. Washington, DC: National Academy Press.
Jung, R.J., Jung, J., (2009). Alcohol, Other Drugs, and Behavior: Psychological Research Perspectives. Los Angeles: Sage.
Krampe, H., Stawicki, S., & Wagner, T. (2006). Follow-up of 180 alcoholic patients for up to 7 years after outpatient treatment: Impact of alcohol deterrents on outcome. Alcoholism, Clinical and Experimental Research, 30 (1): 86–95.
Lawrence, M. V. (2010). What is an alcohol addiction detox? Web.
Leslie, W., Amass, L., Shoptaw, S., Annon, J., Hillhouse, M., Babcock, D., Brigham, G., Harrer, J., Reid, M., Muir, J., Buchan, B., Orr, D., Woody, G., Krejci, J. & Ziedonis, D. (2004). A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction, 100(8):1090-100.
Linnoila, M.I. (1990). Benzodiazepines and alcohol. J. Psychiatr Res., 24. Web.
Löscher, W. & Hönack, D. (1992). Withdrawal precipitation by benzodiazepine receptor antagonists in dogs chronically treated with diazepam or the novel anxiolytic and anticonvulsant beta-carboline abecarnil. Naunyn Schmiedebergs Arch. Pharmacol., 345 (4): 452–60.
Mann, N., Charuvastra, C. & Murthy, K. (1984). A Diagnostic Tool with Important Implications for Treatment of Addiction: Identification of Factors Underlying Relapse and Remission Time Distributions. Substance Use & Misuse, 19(1): 25- 44.
Mattick, R. Lebedeva, R.N., Artamoshina, M.P., & Storozhenko. (2003). Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients. Addiction, 98(4), 441-52.
Mattick, R.P, Kimber, J., Breen, C, & Davoli M. (2002). Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Collaboration, 2.
Midwest Rapid Opiate Detoxification Specialists. (2007). Web.
Nikoda, VV., Lebedeva, R.N., Artamoshina, M.P., & Storozhenko, I.N. (1998). Comparative evaluation of the use of nalbuphine and buprenorphine in prehospital care. Anesteziol Reanimatol., Sep-Oct (5): 23-8.
Office of Applied Studies, Substance Abuse and Mental Health Services Administration. (2010). Overview of opioid treatment programs within the United States: 2008. N-SSATS.
Ries, R.K., Miller, S., Fiellin, D.A., & Saitz, R. (2009). Principles of Addiction Medicine. Philadelphia: Lippincott Williams & Wilkins.
Richter, D., Eikelmann, B. & Berger, K. (2004). Use of the SF-36 in the Evaluation of a Drug Detoxification Program. Quality of Life, 13, (5): 124-132.
Robotham, J. (2005). The great divide over detox. Web.
Strang, J., McCambridge, J., Best, D., Beswick, T., Bearn, J., Rees, S. & Gossop, M. (2003). Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. British Medical Journal, 326: 959-60
Substance Abuse and Mental Health Services Administration. (2009a). Results from the 2008 National Survey on Drug Use and Health: National Findings. (Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434). Rockville, MD.
Substance Abuse and Mental Health Services Administration. (2009b). Substance abuse treatment facility locator. Web.
Teplin, D. Raz, B. Daiter, J., Varenbut, M., Zachos, C. T., Whang, P., Herman, S., Chaudry, S. and Yung, M. (2005). Measurement of symptom withdrawal severity in a 24-hour period after the anesthesia-assisted rapid opiate detoxification procedure. American Journal of Drug and Alcohol Abuse, 31(2):327-335.
Tinkler, E., Catalina, V. B., Brooks, M., Gilbert, J. M., Henderson, R., Shuman, D. J. (2005). Medication-assisted treatment for opioid addiction in opioid treatment programs: Treatment Improvement Protocol (TIP) Series 43. Rockville, MD: SAMHSA’s National Clearinghouse for Alcohol and Drug Information (NCADI).
U.S Department of Health and Human Services (2006). Detoxification and substance abuse treatment. pp. 4–5. Web.
Yakushiji, T., Fukuda, T., Oyama, Y. & Akaike, N. (1989). Effects of benzodiazepines and non-benzodiazepine compounds on the GABA-induced response in frog isolated sensory neurones. British Journal of Pharmacology, 98 (3): 735–740.
Yates, B. T. (2005). Measuring and improving costs, cost-effectiveness, and cost-benefit for substance abuse treatment programs: A manual. Washington, DC: National Institute on Drug Abuse.