Abstract
Opiate dependence is a growing problem in young adults and in patients who have developed addictions while receiving pain management regimens. A major objective of treating patients for their opiate addiction is to suppress withdrawal symptoms while facilitating the detoxification process. Buprenorphine is one of the emerging drugs that has gradually replaced methadone as the drug of choice in opioid detoxification because of safety. Recent studies show that the efficacy of buprenorphine is equivalent to that of methadone when sufficiently high buprenorphine doses are prescribed in combination with rapid induction and flexible dosing. This retrospective study reviewed data of 200 patients who were treated with buprenorphine for rapid detoxification at a medical center. Demographic information such as age and employment status characteristics of the patient were collected. Data were analyzed to determine the efficacy of buprenorphine in reducing major withdrawal symptoms that are most common among opiate drug abusers such as sweating, anxiety, tremor, nausea, and pulse rate.
Introduction
Addiction to opiates is a major risk to the well-being and health of all societies because drug dependence and reliance have been increasing. Statistics show that more than 8% of Americans abuse opiates currently; the most affected age bracket are youths between the age of 20 and 30 who have a prevalence rate of more than 50% (WHO.com, 2009). The use of opiates among youths is well documented as starting between the ages of 10 and 13 with addiction well established by late teens. In fact use of opiates among youths is so rampant that the World Health Organization (WHO) estimates that half of all American twelfth-grade students have used an illicit drug at one time in their lives (WHO, 2009). Ideally, opiates are prescribed legally to relieve pain and because of their addictiveness, they are strictly administered while the patient is under constant observation to avoid addiction. This is because once a person is addicted, it becomes a habit that is virtually impossible to quit.
Continued abuse of opiates leads to destruction and damage of the central nervous system (CNS) by hindering the production of endorphins; it is for this reason that they are among the most dangerously addictive drugs. Continued use of opiates may lead to significantly reduced brain functionality, which happens when nervous tissue becomes dependent on opiates for proper functioning. This dependence results in the typical symptoms that are experienced by opiate drug users when they attempt to withdraw from them such as shaking, chills, and sweating (Doweiko, 2006). Most often, patients experience an array of withdrawal symptoms that can result in more serious forms of physiological symptoms once they attempt to discontinue the use of opiates (Kleber, 2008).
The current literature indicates that buprenorphine is one of the most effective therapeutic methods for treating opiate addictions due to its inherent advantages that surpasses the currently used methadone. A research study by Auriacombe et al. notes one major advantage of using buprenorphine as the drug of choice in treating opiate addiction compared to methadone is that it is unlikely to result in overdose or addiction. This is because of what the authors refer to as “partial agonist at the mu receptor”, which means it is metabolized differently than most standard opiates (Auriacombe, Fatseas, Dubernet, Daulouede and Tignol, 2004).
Buprenorphine
Buprenorphine is a synthetic opioid that has been widely used in detoxification processes in the United States since 2002 to minimize the effects of withdrawal.
However, in Europe the use of buprenorphine has been ongoing for several decades: As early as the 1980’s one practitioner in Europe was already investigating the efficacy of buprenorphine to have it approved as a therapeutic method for treating opioid addiction (Auriacombe et al, 2004). This early research effort eventually bore fruit and buprenorphine was widely used as the standard method of treatment of opiate-addicted cases in Europe by 1996 (Auriacombe et al, 2004).
Buprenorphine is categorized in the phenanthrene class that is made of a hydrocarbon fused with benzene rings. It is not as strong as natural opiates but does have some negative effects such as its ability to cause euphoria. It is, therefore, a form of comprise that physicians accept as worth taking since it provides a high probability of patients recovering from their drug dependence despite these side effects (Marquete, 2008).
Buprenorphine attaches itself to brain cells like natural opioids, binds more strongly than opioids, and hinders natural opioids from reacting; these are the major reasons that make it so appealing as the drug of choice for treating opiate abuse (Magnelli, et al, 2010). Because of this binding ability, buprenorphine can relieve the severe stress and depression that often accompanies opiate withdrawal (Courtwright, 2009).
Buprenorphine is also favored as a method of reducing opiate addiction and dependence because it is a safe method that poses minimal threat to patients.
When patients increase intake of the drug its agonistic effects increase as well. Agonistic effects are directly proportional to the dosage, but with time the patient reaches a plateau phase when the agonistic effects do not increase (Kleber, 2008). This means that if a patient uses excessive amounts of buprenorphine it can never result in dizziness or other drug addiction classical symptoms that are characteristic of opiate drug abuse such as heroin and morphine. Buprenorphine is also convenient because it has a long half-life that makes it possible for the detoxification of patients to be done with one dose every two to three days (Orman and Keating, 2009).
Administration and Metabolism Process
The most common methods of buprenorphine administration are intravenous, intramuscular, and sublingual. The preferred method to ease withdrawal symptoms is by rapid intravenous infusion that takes about three minutes to have the drug distributed throughout the body. Intramuscular administration has a much slower distribution rate that usually takes as long as fifteen minutes. On the other hand, sublingual administration is not preferred as it is cumbersome to administer especially when the patient is in an advanced state of withdrawal; and it has a slow absorption rate that is normally between 200 and 250 minutes (Hales, 2008). Buprenorphine binds tightly to plasma proteins that increase bioavailability and take between 25 and 60 hours to be metabolized in the liver and get fully eliminated from the body. The metabolism process of buprenorphine involves the Cytochrome P4503A enzyme, which is responsible for breaking it down to form norbuprenorphine as a byproduct (Bolourian, 2010).
In the United States, buprenorphine is a relatively new drug that is being used in the treatment of opiate addicts and has not been extensively tested. Because of this, its effects on pregnant women are not yet well known and for safety, purposes are not recommended in this population. It is also not recommended for individuals who are not addicted to opiates because it is itself a form of a mild opiate, and patients who use it may exhibit some of the addictive symptoms of conventional opiates. The focus of this research was to assess the efficacy of buprenorphine in treating opiate addiction withdrawal symptoms.
Methadone was the earliest drug used to treat opiate addiction; it has been in use for over 30 years, and extensive testing and research have established its effectiveness and safety (Hales, 2008). However, its half-life is much lower than that of buprenorphine making it less effective and safe. In terms of chemical metabolism, both methadone and buprenorphine react in the same way by binding onto brain receptors thereby competitively inhibiting opiates from attaching to the same sites (Silk, 2009). This is how most drug addiction treatment drugs can prevent the addictive nature of opiates from being taken up by the body.
More importantly, methadone and buprenorphine are less likely to result in any serious form of dependence, as in the case of opiate drugs, because they get assimilated in the body and metabolize differently than opiates.
Research Design
Various factors are used to determine the effectiveness of any opiate detoxification drug such as the ones that were investigated in this research study. The major variables that were tracked in this research include the ability of buprenorphine to ease and manage various withdrawal symptoms. In this research study, the objective was to examine the effects of buprenorphine on opiate-addicted patients undergoing detoxification.
Research Findings
The study sample included 200 cases randomly selected from the target facility. Data were collected from clinical records. Analysis of data was carried out using the SPSS software program, which assessed the efficacy of buprenorphine in reducing the signs and symptoms of opiate withdrawal.
Demographics
Of the 200 patients whose records were reviewed, 64% were below the age of 40 years while half of all cases were between the ages of 18 and 32. The sample size is typical of the general population as indicated by vast literature in this field. Namely, opiate addiction primarily affects youth who are below 30 years of age. Only 7% of the randomly selected sample was above the age of 60 years. When the cases were analyzed based on occupation, 79% of patients were categorized as working while only 16% were indicated as students.
Symptom management
An ideal detoxification drug is one that is able to reduce the side effects of withdrawal while presenting minimal or little adverse effects to the patient in the process (Cowan, 2008). For this reason the determination of buprenorphine efficacy was examined based on its ability to reduce an array of classical symptoms that are most common among opiate abusers such as increased pulse rate, sweating, chills, restlessness, pupil size, bone and joint ache, running nose and tear production, nausea, vomiting, diarrhea, anxiety, irritability, and yawning. Throughout the research, patients were monitored constantly and data collected at time intervals of 30 minutes, 2 hours and 4 hours after bupenorphine was administered
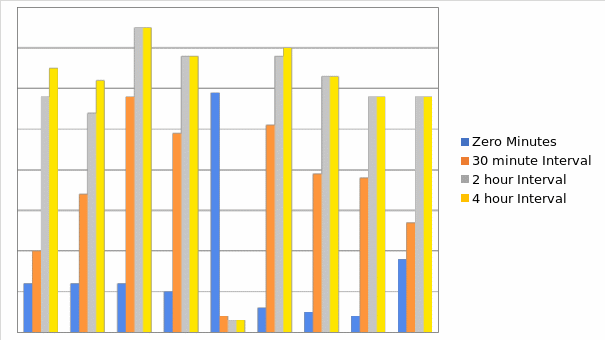
Pulse rates
The data analysis on pulse rates indicated that the majority of cases (39%) had pulse rates that already surpassed the normal range, between 81 and100 at zero minutes, which is just before the bupenorphine was administered, while only 12% had normal rates that were below 80. The other 49% had pulse rates that were neither high enough to be categorized as extremely abnormal, nor low enough to be grouped as normal. At 30 minutes, more cases (20%) had pulse rates below 80, and 42% of the cases had pulse rates below 100. In addition, the patients with the highest pulse rates (above 120) had drastically reduced to less than 10% from 19% at time zero. This reduction in pulse rates indicates that the patients were generally calm and was consistent among patients at every other time interval. At four hours, less than 2% had pulse rate above 120 while the majority (63%) had normal pulse rates below 80. Thus, based on this crucial variable it appears that bupenorphine is effective in attaining the desired physiological change.
Sweating and chills
The number of patients who reported to be free from chills and sweating increased immediately after administration of bupenorphine. At the 30-minute interval, 88% of patients who were suffering from severe sweating had a marked decrease in sweating, and after another two hours an additional decrease in sweating occurred. At four hours more than 60% of patients indicated no symptoms of sweating or chills while only 10% indicated any observable symptoms.
Restlessness
Before the administration of buprenorphine at time zero the majority of patients (72%) were unable to sit still even for a few seconds, and were frequently shifting positions or experiencing extraneous limb movement. Within 30 minutes, symptoms started to ease and 58% of the patients were able to sit still; these changes became even more marked after four hours when up to 74% of the cases indicated the ability to sit still meaning they were not experiencing any form of restlessness. By this time only approximately 25% of the cases still experienced signs of restlessness exhibited through their inability to sit still, however the restlessness was not as pronounced as before bupenorphine was administered.
Pupil size
Buprenorphine was seen to have reduced the number of patients with severe pupil dilation (so that only the rim of the iris was visible) while markedly increasing the number of patients with normal pupil size. At time zero before bupenorphine was administered, less than 10% of the cases had normal pupil size while the rest had pupils that varied in dilation size. At the four-hour interval, as much as 68% of all cases had achieved normal pupil dilation with another 24% having minimal pupil dilation. By this time only 8% of all cases still experienced notable dilated pupils.
Bone / joint aches
Patients reported mild to severe and diffuse joint pain before induction of buprenorphine. In the first 30 minutes after administration of the drug, less than 5% reported any muscle or joint aches reduced from a high of 59%. By the fourth hour, 68% indicated no muscle or joint related symptoms at all with less than 3% reporting any form of muscle or joint aches pains.
Running nose and tear development
The induction of buprenorphine resulted in an increase of patients who claimed not to have running noses and who had only slight nasal stuffiness. There was a decrease in the number of patients who had mild running noses and those who had constant running of noses by 45%. These changes were seen 30 minutes after induction and were more pronounced at 2 hours when up to 75% indicated no such symptoms at all. Four hours after the first dose of buprenorphine was administered fewer patients complained of stuffy noses as compared to before the drug was administered. There was a large reduction in the number of patients who had constant running noses from 79% to 6%, as compared to before the drug was administered.
Effects of buprenorphine on the gastrointestinal tract
Within 30 minutes after the initial dose of the drug there was a reduction in the number of patients complaining of vomiting and multiple diarrhea episodes from 140 cases to only 25 cases. At two hours, the efficacy of the drug continued and there was an additional decrease in nausea and multiple diarrhea cases. The effects of the drug stabilized at four hours and were similar to those recorded at two hours where more than 60% of the cases experienced no gastrointestinal symptoms and less than 5% reporting any form of GI symptoms. What is notable is that the effects of buprenorphine on nausea and vomiting impulses at four hours reduced significantly after four hours implying a causal-effect association.
Anxiety and irritability
Thirty minutes after the induction of the drug, there was a decrease in the number of patients who were obviously irritable or severely irritable from 165 to 51. At three hours, there was a substantial decrease in irritability levels of the patients with 58% of the patients not being irritable at all and 30% being slightly irritable. By this time only 12% of patients were still obviously irritable or extremely irritable.
Yawning
Yawning does not have any harmful effect for patients but it is a useful tool to study the effects of buprenorphine during opiate detoxification, as yawning is a common and annoying withdrawal symptom of opiate addiction that is easily observed by the clinician. After the administration of the first dose of buprenorphine there was considerable change in the yawning characteristics of the patients. Data collected at 30 minutes after the first dose shows some change in yawning behavior of the patients. At four hours, the number of patients who were yawning, yawned once or twice during the interview, yawned three or more times and those who yawned multiple times had drastically reduced. There was an overall increase in the number of patients who did not yawn during the interview from 27% to 57%.
Chi-square analysis of the data was done to determine the effectiveness of the chosen hypothesis. Chi-square enables researchers to test the validity of observed results from expected results. In this study, there was a 5% chance that the observed readings were incorrect. The alternative hypothesis of this study was there is a correlation between the patient effects observed and the detoxification procedures used at the detoxification centre. While the null hypothesis was that there is no correlation between the patient effects observed and the opiate detoxification procedures used at the detoxification centre. Based on the chi-square figures the alternative hypothesis was accepted.
Discussion
Buprenorphine has a half-life of between 25 hours and 72 hours. The drug is able to ease severe symptoms that often accompany opiate withdrawal. The main buprenorphine drug that was used in the inpatient center was Suboxone. This is a reliable drug that has been found to have all the characteristics of a generic bupenorphine drug with similar results when administered to patients. Buprenorphine is also administered in small but effective doses of 8mg per patient, and only in rare cases is 16mg dosage given; at 8mg dosage the efficacy of the drug has been found to be reliable in reducing the classical symptoms of opiate withdrawal. A small percentage (2%) of all the patients in the study required 16mg doses which indicated that small doses of buprenorphine were ineffective in treating withdrawal symptoms for this category of patients.
Buprenorphine was found to have notable effects on reducing opiate addiction withdrawal symptoms. The use of buprenorhine in the detoxification procedure resulted in a reduction in the number and severity of symptoms experienced by the patients. There was an interesting trend among the demographic characteristics of the cases from which data were collected during the research study. Given that the sample size was randomly selected, we have to assume that the study sample selected in this case is a fair representation of the larger study population that is being studied.
The majority of the cases in the current study were employed (79%). This could indicate that this category of persons had stable incomes, which means they were able to fund their addiction. Additionally, half of all cases being treated for opiate addiction were between the age of 18 and 32. In addition to having stable incomes, they may have fewer responsibilities and be more predisposed to be adventurous.
In each of the variable for which the efficacy of bupenorphine was measured among patients, each indicated that the drug was able to markedly reduce opiate withdrawal symptoms. In fact, for the nine different variables on which this research study focused, buprenorphine was seen to reduce and stabilize more than 40% of the cases in each symptom. The range of symptoms that were monitored during this research study are most relevant since they are the ones that are found to be most common among the majority of patients undergoing opiate withdrawal treatment. One of the most important variables that the research tracked during the study was the recording of pulse rates. The relevance of this variable is that pulse rate is an important indicator of a range of other typical symptoms including the overall health status of an individual (Hales, 2008).
A snapshot of the patients pulse rates at zero minutes indicate that majority of patients (90%) had pulse rates that were already above normal. Four hours after buprenorphin was administered 63% had normal pulse rates while only 2% were still experiencing abnormally high pulse rates. If these findings are anything to go by then we would expect a drastic reduction on a range of similar symptoms among patients in similar proportions. This is because pulse rates are often an indicator of a person’s physiological condition. This is because with reduced pulse rate, a person is less likely to sweat, experience chills, have muscle pains or be irritated because the emotional and physiological aspects are stabilized and thereby functioning properly.
Indeed, as the record results indicate, there was improvement in all the other symptoms. For each of the variables being monitored, a reduction of the specific symptom was achieved by the fourth hour following administration of bupenorphine. For instance, there is a reduction of patients experiencing sweating and chills shortly after they were administered bupenorphine, which continued at 4 hours. In fact, except for two types of symptoms that were observed; yawning and anxiety and irritability, all other symptoms showed improvements that were above 60%. Of all the symptoms observed, irritability was found to be the most drastically reduced among patients after four hours from the time bupenorphine was administered while yawning was the least as indicated in the data analysis section. Hence, even from a rough estimate based on all variables measured we can determine the efficacy of bupenorphine in the treatment of opiate addiction withdrawal symptoms.
Conclusion
This research study supports the use of buprenorphine as a therapeutic method of treating opiate addiction because of its efficacy in reducing the associated withdrawal symptoms. The most significant effects noted in this study were decreasing pulse rates, reducing sweating, easing pupil dilation, preventing the formation of joint pains in normal patients and easing the pains in those who are already suffering from pain. It was also seen to be effective in normalizing running nose and tear production, easing of nausea and vomiting, reducing anxiety and restlessness and easing of tremors. In conclusion the data collected from this study show that buprenorphine is effective against most withdrawal symptoms and can be effectively used to treat these symptoms for prolonged periods of time.
References
Auriacombe, M., Fatseas. M.,Dubernet, J., Daulouede, J. & Tignol, J. (2004). French Field Experience with Buprenorphine. The American Journal of Addictions, 13(1): 17-28.
Bolourian, S. (2010). Buprenorphine: A Guide for Readers. New Jersey, NJ: Dane Publishing.
Courtwright, D. (2009). Dark paradise: a history of opiate addiction in America. New York, NY: Harvard University Press.
Cowan, A. (2008). Buprenorphine: combatting drug abuse with a unique opioid. Michigan, MI: Wiley-Liss.
Doweiko, H. (2006). Concepts of Chemical Dependency. California, CA: Cengage Learning.
Hales, R. (2008). Study Guide to Substance Abuse Treatment. Arlington, VA: American Psychiatric Publishing.
Junig, J. (2009). User’s Guide to Suboxone: Taking buprenorphine for opiate Dependence. Wisconsin, WI: Terminally Unique Publishing.
Kleber, H. (2008). The American Psychiatric Publishing textbook of substance abuse treatment. Arlington, VA: American Psychiatric Publishing.
Langrod, J. (2008). The Substance Abuse Handbook. Philadelphia, PA: Lippincott Williams and Wilkins.
Magnelli, J., Biondi, B., and Calabria, K., (2010). Safety and efficacy of buprenorphine/naloxone in opioid dependent patients. California, CA: Clinical Drug Investigations.
Maremmani, I. (2010). Buprenorphine-based regimens and methadone for the Medical treatment of opiate addiction. New York, NY: Med Publishers.
Marquete, P. (2008). Buprenorphine Therapy of Opiate Addiction. New Jersey, NJ: Humana Press.
Millman, R. (2007). Substance abuse: a comprehensive textbook. Philadelphia, PA: Lippincott Williams and Wilkins.
Orman, J., and Keating, G. (2009). Buprenorphine/Naloxone: a review of its use in treatment of opiate addiction. New Jersey, NJ: Humana Press.
Renner, J. (2009). Handbook of Office-Based Buprenorphine Treatment of Opioid Dependence. Arlington, VA: American Psychiatric Publishing.
Silk, K. (2009). Cambridge Textbook of Effective Treatments in Psychiatry. New York, NY: Cambridge University Press.
Strain, E. (2005). The treatment of opioid dependence. Maryland, MD: John Hopkins University Press.
Thakkar, V. (2008). Addiction. New York, NY: Infobase Publishers.
WHO.com. (2009). Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva: World Health Organization.