Introduction
The production and purification of proteins is a difficult and expensive task which takes time. Naturally, proteins are produced by plants and animals from their building blocks known as amino-acids. However, this is a slow process and the products are usually very limited and in small amounts which cannot meet the industrial needs, say for example the production of enzymes for industrial use.
This has challenged scientists to come up with several methods that can be used in the mass production of the proteins for industrial and large scale use in daily life. These include the production of enzymes, medicines and food supplements among other uses. One of the major ways that protein production is carried out is by expressing them in plants. β-glucuronidase is one such protein that is manufactured in large scale for industrial use as an enzyme.
This protein, β-glucuronidase is prepared industrially by expressing in transgenic plants like tobacco (Menkhaus et al., 2004), as a recombinant protein, which is then later extracted and purified by several methods before it is ready to use. The amount of protein that is recovered from this process is sufficient enough to cater for the industrial needs of a certain activity, for example enzyme production.
Plant expression systems have turned out to be a very good option for the mass production of the proteins used for medicinal, pharmaceutical and commercial purposes too.
These plant expression systems, using transgenic plants, are beneficial and have some advantages including the low production cast that is involved, high and fast rate of production of a certain protein (Larrick et al., 2001), safety in that the plants do not produce harmful toxins which may be harmful to the humans and plants have also been shown to produce a large variety of recombinant proteins in their systems which may be later purified (Fischer and Emans, 2000).
The most commonly used plant for the industrial production of β-glucuronidase is the Tobacco plant. This transgenic plant is considered most suitable since there cannot be a transfer of any harmful protein produced by the plant to animals or humans because it is neither a feed crop nor a food crop (Fischer et al., 2004). Furthermore, the tobacco plant has in place strong mechanisms and regulatory control measures that can be used for the expression of transgene proteins.
Another advantage of the tobacco plant is that it produces a large biomass which makes it a very suitable plant for the production of the recombinant β-glucuronidase in large quantities. One disadvantage, however, of the tobacco plant as a transgenic option is that it produces large quantities of natural plant proteins and phenolic and alkaloid compounds which hamper the purification of the expressed recombinant proteins.
Uses of β-glucuronidase
Some of the uses of the β-glucuronidase in the industry include as a component in diagnostics. The β-glucuronidase is an enzyme which has the abilities to carry out the splitting of compounds that contain glucuronic groups. These glucuronic groups are mainly present in the spleen, liver and also in the reproductive and endocrine tissues of some higher animals and mammals alike.
Thus in diagnostics, the β-glucuronidase is used in the determination of the amount of steroids and or proteins which contain the glucuronic compounds in blood. In addition to this function, the β-glucuronidase is used as an iso-enzyme in the molecular biology assays mainly as a reagent.
Purification of β-glucuronidase
The purification of the recombinant β-glucuronidase from the transgenic plant proteins is essential for the proper adsorption and functioning without impurities. Some of the impurities that have to be eliminated from the transgenic plant extract for example from the tobacco plant include acidic elements of phenolic acids and phytic acid, native plant proteins, nucleic acids in addition to the nicotine present which has toxic alkaloid properties.
These form complexes with the recombinant proteins thereby making processes such as column chromatography to be interfered with. In order to eliminate this, the inclusion of certain elements such as beta mercaptoethanol or dithiothreitol in the extraction process helps to increase the amount of recovered recombinant protein from the transgenic plants. A phenolic-binding agent such as polyvinyl polypyrrolidone may also be included to decrease the interference brought about by the phenolic compounds from the extract (Holler et al., 2007).
Extraction
The first step of is the extraction of the recombinant protein fro the transgenic plant extract. This is done by the use of extraction buffer made of 50mM Sodium Phosphate, 10mM 2-mercaptoethanol, and 1mM EDTA pH 7.0. This is in the aqueous two-phase extraction (ATPE) method which is a very powerful and resourceful technique that has been functional in facilitating bio-catalytic reactions (Spiess et al., 2008).
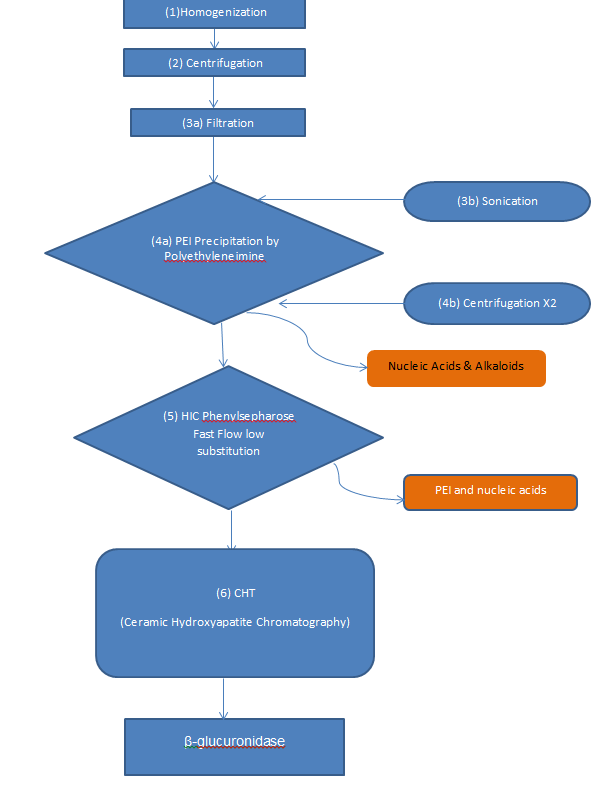
Homogenization
Homogenization is then carried out until the sample contains no large particles in the mixture. A 2% w/v polyvinylpolypyrollidone that was pre-hydrated is then immediately added to the mixture and centrifuged at 17000xg and left to stand for about fifteen minutes at room temperature and pressure. The supernatant is then removed and filtered through a syringe.
Polyethyleneimine (PEI) precipitation
Polyelectrolyte precipitation then follows whereby the Polyethyleneimine diluted to 10mg/ml in deionized water and adjusted to a pH of 7.0. This is added to the extract in a ratio of 800mg of the PEI per total protein extracted. Upon addition of the electrolyte, the samples are centrifuged vigorously for about 15 seconds and allowed to stand at room temperature and pressure for about half an hour. After this, they are then further centrifuged for about 20 minutes at 17000xg and the supernatant removed for analysis.
The pellets are then suspended in a resuspension buffer consisting of 50mM NaPi at pH 7, 10 mM BME, 1mM EDTA and 0.5M NaCl. Sonication is then done for about 5 seconds and then followed by centrifugation at 17000xg for 10minutes. The supernatant is removed and the samples again centrifuged at 16000xg for 10minutes.
HIC Chromatography
Chromatography of the supernatants then follows as the next step in the purification process. Phenyl Sepharose Fast Flow low substitution is used in this process. The column is packed to approximately 5.1 cm bed volume and an equilibrating buffer comprising of 50mM NaPi at pH 7.0 and 1.5mM Ammonium Sulphate buffers. The proteins are eluted in a linear gradient and aliquots of 2mL collected.
Ceramic Hydroxyapatite Chromatography
Ceramic hydroxyapatite is then packed into a column as slurry in a bed height of about 10cm with the equilibrating buffer being 40mM NaPi at pH 6.8. The proteins are eluted in a linear gradient as the eluting buffer. 1mL fractions are collected per minute and pooled for concentration.
Size exclusion chromatography can also be employed in the separation of the recovered proteins. These have different molecular weights and sizes and therefore can be separated by this method. This is because the natural proteins from the tobacco plant namely Rubisco consisting of two subunits, a smaller one of about 13 kDa units and a larger one of about 55 kDa units. The β-glucuronidase recombinant protein however is much larger than these two: it is about 68 kDa units.
Rubisco is basically an acidic protein with an isoelectric point of about 6.0. This property therefore creates a challenge in the purification and separation procedure. This is because the recombinant β-glucuronidase protein is also acidic in nature and separation processes such as ion-exchange chromatography are futile in the purification process.
The Purification Process
The purification process after extraction mainly incorporates 3 stages namely Polyethyleneimine precipitation, HIC Chromatography and Ceramic hydroxyapatite chromatography. In the first stage of purification, the Polyethyleneimine acts to bind the nucleic acids and the alkaloids that might have been extracted together with the recombinant β-glucuronidase proteins. This stage has a pH of 7 in order to prevent the destruction of the extracted proteins.
The HIC stage mainly functions to remove the PEI and any nucleic acids that might have passed through the first stage. This is made possible by the phenyl Sepharose binding to the PEI at the pH of 7.0. and the nucleic acids present. The last stage is basically a polishing stage where the eluted β-glucuronidase is isolated and obtained in a much pure form and whose activity is higher.
Conclusion
From the above process, the acidic recombinant β-glucuronidase protein can be recovered and efficiently purified from the transgenic tobacco plant extracts. The PVPP and BME serve to eliminate the impurities in the extract so that the adsorption in the subsequent chromatographic stages can be increased. This also increases the yield of the recombinant β-glucuronidase protein form the extract. The Polyethyleneimine precipitation phase serves as a step in the elimination of the initial impurities such as nucleic acids and alkaloids.
The HIC step is effective in the removal of the PEI and nucleic acids resulting from the first step in the purification process. The HAC step acts as the polishing stage of the products. In this process, approximately 41% purity of the initial β-glucuronidase protein can be recovered. The choice of using ceramic hydroxyapatite (HA) resin is due to its unique ability to bind acidic proteins.
Hence, it is employed in the removal of the acidic proteins by binding them and eliminating them from the mixture. These can then be removed from the extract/ supernatant through the fractionating column. Furthermore, it has the potential of scaling up the purification process. The HA resin which is mainly 10mM NaPi requires a low salt sample to bind the proteins.
The purity of the recovered protein can be assayed by use of Sodium Dodecyl Sulphate (SDS-PAGE) analysis. Here, the products are run through the SDS-PAGE gel and stained with Coomasie Brilliant blue stain and later assayed by staining with silver stain.
References
Fischer, R. and Emans, N. (2000). Molecular Farming of Pharmaceutical Proteins, Transgenic Res. 9, pp. 279–299.
Fischer, R., Stoger, E., Schillberg, S., Christou, P. and Twyman, R.M. (2004). Plant-based Production of Biopharmaceuticals, Current. Opinion. Plant Biol. 7, pp. 1–7.
Holler, C., Vaughan, D. and Zhang, C. (2007). Polyethyleneimine Precipitation versus Anion Exchange Chromatography in Fractionating Recombinant β-glucuronidase from Transgenic Tobacco Extract. J Chromatogr A 1142(1), pp. 98–105.
Larrick, J.W., Yua, L., Naftzgera, C., Jaiswala, S. and Wycoffa, K. (2001). Production of Secretory IgA Antibodies in Plants, Biomol. Eng. 18 (3), pp87–94.
Menkhaus, T. J., Bai, Y., Zhang, C., Nikolov, Z. L., Glatz, C. E. (2004). Considerations for the recovery of recombinant proteins from plants. Biotechnol Prog 20(4):1001–1014.
Spiess, A.C., Eberhard, W., Peters, M., Eckstein, M.F., Greiner, L., Büchs, J. (2008). Prediction of Partition Coefficients using COSMO-RS: Solvent Screening for Maximum Conversion in Biocatalytic Two-phase Reaction systems, Chem. Eng. Process. 47, pp. 1034–1041.