Nitrate, also known as Nitrogen trioxide is an odorless, colorless, and flavorless compound that is found in some groundwater, soil, and some foods. The chemical equation for nitrate is NO3 or alternatively NO3-N (nitrate-nitrogen) and has a molecular mass of 62.0049 g/mol. Nitrates are chiefly used as a major ingredient in fertilizer. They are also used to make explosives and glass. For example, potassium nitrate is used as gun power. Furthermore, nitrate compounds are important in chemical production processes and industrial separation processes (Clarke et al, 2002, p. 45).
Nitrites have a molecular weight of 46.0049 g/mol and their chemical equation is NO2. They are mainly utilized as food preservatives, for example, Sodium nitrite is used as a food stabilizer, color fixative, antimicrobial agent, and flavoring agent in treated meats and foodstuffs. However, In the environment, nitrates are easily transformed into nitrites and vice versa. Both nitrates and nitrite are compound ions with a negative charge (anions). They have a propensity of uniting with ions with a positive charge (cations), to attain a neutral charge state (Clarke et al, 2002, p. 45).
Nitrates are nontoxic compounds, however, when converted to nitrites in human bodies, depending on the level of nitrate concentration they can cause cyanosis, weakness and a rapid pulse, coma, and cancer. These side effects to a large degree affect adults who have little or lack methemoglobin reductase enzyme and also those with inadequate stomach acid (vegetarians). As of these adverse effects, the Food and Drug Administration permits these compounds to be utilized as food additives provided they are of food standard and are added not more than the quantity needed. Currently, the highest amount of nitrite permitted in cured meat and fish is 200 ppm (Clarke et al, 2002, p. 45).
Nitrate and nitrite are chemical compounds that have a nitrogen atom linked to oxygen molecules; nitrate is a conjugate base of nitric acid and has one inner nitrogen atom enclosed by three indistinguishable oxygen atoms in a trigonal planar array while nitrite has two oxygen atoms. The resonance structures of nitrate are as follows:
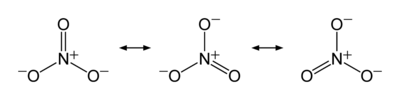
While for Nitrite, the molecule structure is; O=N=O. As depicted in their structures, both nitrate (NO3) and nitrite (NO2) are negatively charged and they are likely to unite with positively charged, to attain a neutral charge state which is stable (Cemek et al, 2006, p. 23).
Nitrate (NO3) occurs naturally as soil nitrogen. In the normal nitrogen cycle, bacteria transform nitrogen to nitrate, this is consumed by plants and integrated into tissues. These plants are eaten by animals and later nitrate is cycled back to the environment in animal feces, additionally by microbial degradation of animals and plants once they die. Nitrate can be converted to nitrite by microorganisms; this reaction takes place in the atmosphere, the digestive tract of human beings, and other animals. Once nitrate has been reduced to nitrite in the environment by bacteria, the nitrogen cycle is finally completed when nitrite is converted to nitrogen. This shows how nitrates and nitrites are found in food. Furthermore, these compounds are found in the groundwater which is consumed by human beings (Cemek et al, 2006, p. 23).
Nitrate is a standard constituent of the human being’s diet, with normal everyday eating from all sources approximated at 75 milligrams (mg). Upon intake, roughly 5% of the nitrate consumed by healthy adults is reduced to nitrite by microorganisms in saliva. Further nitrogen trioxide is converted by microorganisms within the alimentary tract. When the pH of the gastric fluid in the stomach is above 5, it supports the development of nitrate-reducing microorganisms thereby increasing the rate of conversion of nitrate to nitrite. This process leads to cyanosis (blue baby syndrome) in infants, whose gastrointestinal systems commonly have a greater pH as compared to those of adults. The blue baby syndrome occurs when the nitrite level increases to 10%. In addition, when nitrite reacts with food protein it forms N-nitroso compounds which and these compounds formed are carcinogenic in test animals that are cancer-causing. The debate on their potential to cause cancer has not been concluded. Finally, long Long-term contact with minor levels of nitrates and nitrites can lead to diuresis. This is a body condition where there is an increase in the starchy deposits and quantity of urine, and bleeding of the spleen (Cemek et al, 2006, p. 23).
Because of these risks associated with nitrates and nitrite, toxicity values of these compounds have been developed by U.S. Environmental Protection Agency(EPA) to approximate the danger of non-cancer health effects from intake of nitrates and nitrites. The toxicity value employed to approximate non-cancer consequences after ingestion is known as reference dose (RfD). An RfD is approximate the maximum amount that can be consumed daily without leading to an adverse effect. The reference dose for nitrate was developed bearing in mind the concentration at which methemoglobinemia showed levels higher than 10% for 0- to 3-month-old infants. This was founded on daily basis ingestion of recipes made with water having 10 mg per liter (mg/L) of nitrate in the form of nitrogen. For nitrite compound, the reference dose (RfD) is founded on a 10-kg (22-pound [lb]) infant drinking 1 liter, or approximately 1 quart, of water on a daily basis. The RfD stands for a “safe daily dose” and therefore is weighed against the amount an individual is approximated to ingest on a daily basis, as a ratio. Ironically, there are no known adverse effects connected with breathing in nitrates or nitrites, therefore no reference concentrations (RfCs) to evaluate inhalation toxicity. However, role played by nitrites, and not directly nitrates, to possible human carcinogenicity and the scale of the linked danger are uncertain (Cemek et al, 2006, p. 23).
Source: Argonne National Laboratory, EVS Human Health Fact Sheet, August 2005.
Environmental Protection Agency (EPA) limits the amount of sodium nitrite released in the environment not to be more than 100 lb (45.4 kg) and nitrate emission not to be more than 10,000 lb (4,540 kg). All these emissions are reported and recorded in the countrywide Toxics Release Inventory. EPA further gives a 1-10 pound limit for nitrosamines, 10ppm for nitrate, and 1ppm for nitrite for drinking water. Finally, Food and Drug Administration permits nitrate and nitrite to be used as food preservatives within a limit of 200ppm for cured meat and fish 200ppm (Argonne National Laboratory, 2005, p. 17).
Two independent case studies were carried out to determine the level of nitrates and nitrite in food and their effects. We will discuss the two case studies independent.
- Case 1: Nitrate and nitrite levels in fruity and natural mineral waters marketed in western Turkey.
- Case 2: Levels of phytoestrogens, inorganic trace-elements, natural toxicants, and nitrate in vegetarian duplicate diets.
Reference List
Argonne National Laboratory EVS (2005). Nitrate and Nitrite: Human Health Fact Sheet, 1-2.
Cemek, M., Levent, A., Yavuz, O., Kamil, S., Sait, B., Muhsin, K. (2006). Nitrate and Nitrite levels and natural mineral waters marketed in western Turkey: Journal of Food Composition and Analysis , 20, 238-240.
Clarke, B., Karen, A. , Laurence, C., Martin, R., Lesley A., Malcolm, J., Keith R., DuPont, M. (2002). Levels of phytoestrogens, inorganic trace-elements, natural toxicants and nitrate in vegetarian duplicate diets: Food Chemistry, 81, 287-300.
MAFF. (1998). Total diet study—nitrate and nitrite, Food Surveillance Information Sheet 163. London, UK: UK Ministry of Agriculture, Fisheries and Food, HMSO.
Walker, R. (1990). Nitrates, nitrites and N-nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food Additives and Contaminants, 7(6), 717–768.
Zanders, J. M., Hedley, M. J., Pakmer, A. S., Tillman, R. W., & Lee, J. (1999). The source and distribution of cadmium in soils on a regularly fertilised hill-country farm. Australian Journal of Soil Research, 37(4), 667–678.