Introduction to Osmosis
Osmosis is one of the important ways that plants and animals achieve homeostasis (Djelti 48). It is the process of moving water through a semipermeable membrane from an area of low osmolarity to an area of high osmolarity. Osmosis plays a vital role in the body of human beings, particularly in the kidneys and the gastrointestinal system (Djelti 48). It helps the body absorb food nutrients and eliminate waste products such as urea and excess salts from the bloodstream. Therefore, understanding how an egg reacts when placed in solutions of different concentrations enables one to understand the role of osmosis in the human body.
In understanding the osmosis process, one needs to familiarize oneself with the difference between a solution, solute, and solvent. For instance, a solution is a homogeneous mixture containing one or more dissolved solvents. A solvent is a substance that can dissolve a solute to form a homogeneous mixture (Savva 160). A solute is a substance that can dissolve in a solvent (Savva 164). Therefore, chemicals that can dissolve in a solvent are considered soluble. Hence, a solution is formed when one or more solutes dissolve into a solvent. For instance, salt and sugar are substances that are soluble in water.
A solution is considered hypertonic if it has more concentration of solute particles. On the contrary, a solution containing fewer dissolved particles is considered hypotonic (Savva 164). Solutions whose solute concentration is equal to that of the cell are said to be isotonic. In the body of a human being, osmosis occurs in various places, such as the kidneys and the gastrointestinal system (Djelti 49). Osmosis plays a significant role as it helps the body absorb nutrients from food and eliminate waste products from the bloodstream (Djelti 49). It also stabilizes the internal movement of water and the intercellular fluid levels within the cell.
Water does not move inside or outside the cell when the solution is isotonic. This happens because there is both the cell and the solution outside have equal particle concentrations. Hence, water molecules cannot move from one side of the cell to the other (Vujovic et al., 628). As a result, the cell does not change its size and shape. However, when the solution is hypotonic, there is a net flow of water towards the cell. This water movement to the cell is because the solution has less particle concentration than the cell. Therefore, water molecules move inside the cell, change their size, and increase their volume (Vujovic et al., 630). A cell in a hypertonic solution shrinks because water moves from the cell. The solution contains more dissolved particles. Thus water molecules get drawn from the cell.
Methods for Osmosis
Six eggs were placed in a bowl full of vinegar to cover them. They were left inside vinegar for 48 hours until their shell dissolved completely. They were then gently washed using tap water to remove any remaining shells. Data of the eggs were collected that comprised weight measurements and pictures to help in differentiating them throughout the experiment. The eggs were divided into three pairs, each placed in a bowl of corn syrup, distilled water, and fresh vinegar. However, each egg was in a separate container, but the solution was the same. The eggs were left for another 48 hours. Finally, they were removed from the solutions and new measurements recorded. The egg observations were done and discarded.
Image of Egg when Dissolving Shell in Vinegar Solution



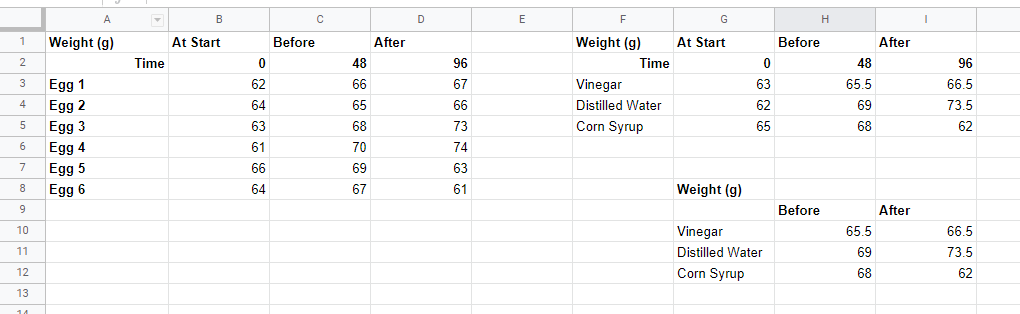
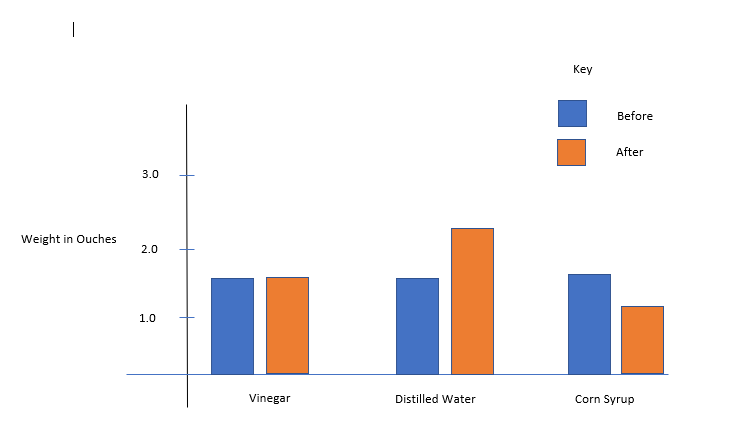
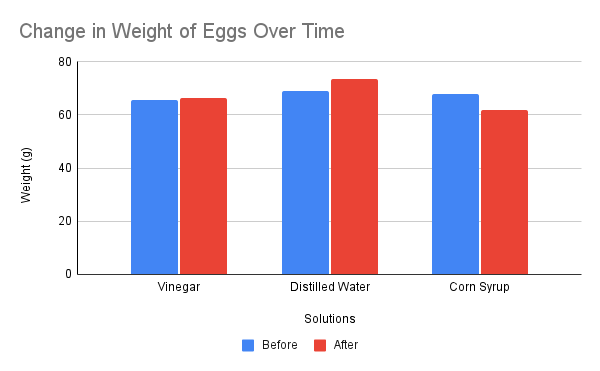
Throughout the experiment, the eggs in the corn syrup solution decreased in size and shape because the corn syrup solution has high solute concentration than the egg. Hence, water moved from the egg into the syrup and made the egg shrivel (Saiba 210). There was no nay observable change in the eggs in vinegar because there was equal particle concentration on both sides. Consequently, no water movement occurred between the egg and the solution. As a result, the eggs maintained their shape and size (Saiba 211). Finally, in the case of distilled water, the egg had a high solute concentration. Therefore, water moved inside the egg increasing its size and volume.
Discussion for Osmosis – Draft
The study supported the hypothesis because the results revealed that cells react differently when placed in a different solution. For instance, when the surrounding solution was hypertonic, water moved from the cell and made it shrink (Vujovic et al., 628). When the cell was in vinegar, it maintained its shape because both the egg and the solution had the same solute concentration (Vujovic et al., 628). Finally, in a hypotonic solution, the cell had more particle concentration than distilled water hence water moved to the cell and increased in size.
Vinegar solution was an isotonic solution because there was no water movement either from the egg to vinegar or from vinegar to the egg. This indicates that the solute concentration between the two sides was equal. Hence, vinegar must have been an isotonic solution. Distilled water was a hypotonic solution because water molecules moved to the egg (Vujovic et al., 632). This shows that distilled water had low solute concentration than the egg causing water to flow inside the egg. Finally, corn syrup was a hypertonic solution because water molecules moved from the egg to the corn syrup solution (Vujovic et al., 632). This indicates that there was more solute concentration on the corn syrup than on the egg forcing water molecules to move from the area of low concentration to that of high concentration. This is an indicator that corn syrup is a hypertonic solution.
This experiment can be expanded by submerging both eggs in vinegar to regain their shape. The eggs regaining back their original shape will make the students better understand how osmosis works (Vujovic et al., 632). This is because they might think the egg shrank because of a hole in its membrane unless the membrane is shown to be intact (Vujovic et al., 632). Therefore, this will only be possible if the shape of the egg shape is regained.
Results from these egg experiments reveal that osmosis is essential to the human body. This is because all cells in the human body need the right amount of water inside to maintain their shape, give energy, remove waste products and perform other activities to keep the body healthy. This is why medicines injected into the bloodstream have an equal concentration to body cells (Saini 294). If a person becomes sick and is dehydrated, they get a 0.90% saline IV drip (Saini 294). If such a solution happens to be slightly different from this, it would not be isotonic anymore. It might make the blood vessels contract or even explode because of the concentration of the dissolved solutes in water (Saini & Rummi Devi 294). Therefore, osmosis in the body occurs the same way as it was observed in the egg experiment.
Works Cited
View. “Fun Science Experiment – Dissolve an Egg Shell.” Untamed Science. N.p., 2021. Web.
Djelti, Samir. “Osmosis: the unifying theory of human migration.” Revue Algérienne d’Economie et de Management 8.2 (2017): 48-59.
Saini, Rummi Devi. “Health Risks from Long Term Consumption of Reverse Osmosis Water.” Int J Applied Chem 13 (2017): 293-301.
Savva, Michalakis. “Isotonic Solutions.” Pharmaceutical Calculations. Springer, Cham, 2019. Pp. 157-180.
Saiba, Ali. “In Desalination, from 1987 to 2009, the birth of a new seawater pretreatment process: Electrocoagulation-an overview.” Desalination and Water Treatment 16.1-3 (2010): 201-217.
Vujovic, Predrag, Michael Chirillo, and Dee U. Silverthorn. “Learning (by) osmosis: an approach to teaching osmolarity and tonicity.” Advances in physiology education 42.4 (2018): 626-635.