Introduction and Literature Review
When temperature decreases, liquid changes into solid, which is called freezing. The temperature at which the solid-liquid change takes place depends on the chemical composition of the substance (Ge & Wang, 2009b). While pure compounds melt and freeze at certain temperatures, the addition of other substances may alter the freezing temperature; that is, the resulting mix will often freeze at a different temperature (Ge & Wang, 2009a).
The process of freezing of water occurs as described in the literature. When the temperature of water lowers, the speed of water molecules decreases (Eaton, 2011). At a certain point, the molecules begin moving so slowly that they stop being able to escape intermolecular attraction forces; clusters of molecules start forming in the water, and they attract even more molecules of water, which causes its crystallization and freezing (Eaton, 2011).
The presence of some compounds, such as salt, may disrupt the process of formation of crystals, because the particles of salt will be present between different water molecules, and they will block the access of other water molecules, thus preventing the formation of clusters, and, consequently, freezing (Eaton, 2011). It is also clear that the concentration of the additional compounds affects the freezing point of water – when more particles are present, they block the access of more water molecules to one another (Ge & Wang, 2009b; Eaton, 2011). Importantly, only the quantity of particles (and not their nature) that are solved in water will affect the freezing point of water, which also means that, e.g., n moles of a compound that dissolves by breaking into ions (e.g., salt, NaCl) will lower the freezing point of water more than n moles of a compound that does not break into ions (e.g., sugar; Bewick, Parsons, Forsythe, Robinson, & Dupon, 2017).
The fact that the temperature at which a liquid freezes drops when additional substances are solved in the liquid is called the freezing point depression (Ge & Wang, 2009b). The freezing point depression means that a water solution will take more time to freeze because it will be needed to cool it to a lower temperature (Bewick et al., 2017). In addition, solving substances in water also changes the liquid’s heat capacity, which means that a different amount of energy will need to be lost by the liquid to lower the temperature of a given amount of that liquid by 1 degree; this also may affect the rate at which the water solution will freeze.
Objectives
The objective of the experiment will be to find if the freezing rate of water changes when different substances are added.
Hypothesis
The hypothesis for the current experiment will be that adding different substances to water will affect its freezing rate (i.e., the amount of time it will take the liquid to freeze). As explained above, the particles of the solved substances should be expected to lower the freezing point of water, and because the concentration of new substances will be different in various solutions, the freezing rte may change differently. Changes in the freezing rate may also occur due to the varying heat capacity of different solutions.
Materials
The materials used in this experiment are as follows: 4 bowls with 1 cup of water in each; 1 tablespoon of honey; 1 tablespoon of salt; 1 tablespoon of apple cider vinegar; toothpicks; a freezer with the temperature of 28 degrees Fahrenheit (-2.2°C).
Methodology
Variables
Main variables
The independent variable of the experiment is the substance added to water (none; honey; salt; apple cider vinegar). The dependent variable is the rate of freezing. The rate of freezing is measured in minutes, although measurements will only be taken every five minutes; the time will be measured using an electronic alarm clock, and the state of water will be recorded on paper at each point.
Confounding variables
As for confounding variables, it should be noted that the temperature in the freezer will be altered every five minutes when the freezer will be opened to check if the liquids have frozen. Nevertheless, this should not be a barrier to fulfilling the objectives of the experiment, because all the bowls will be put into the same freezer, so the changes in the temperature inside the freezer will affect each of the bowls equally, which will mitigate the potential disruption form this confounder. Another confounder is related to the fact that the water that will be used may not be completely pure, but may contain some dissolved substances. This should not affect the results of the experiment severely, because the same type of water will be used in all bowls.
Methods
Four bowls of water were taken (1 cup of water in each). Into three of the bowls, 1 tablespoon of different substances was added into each: honey to the first bowl, apple cider vinegar to the second bowl, and salt to the third bowl. Then, they were put in the freezer with a temperature of 28 degrees Fahrenheit. After every 5 minutes, each solution was checked by putting a toothpick in the water to see if it was already frozen.
Results
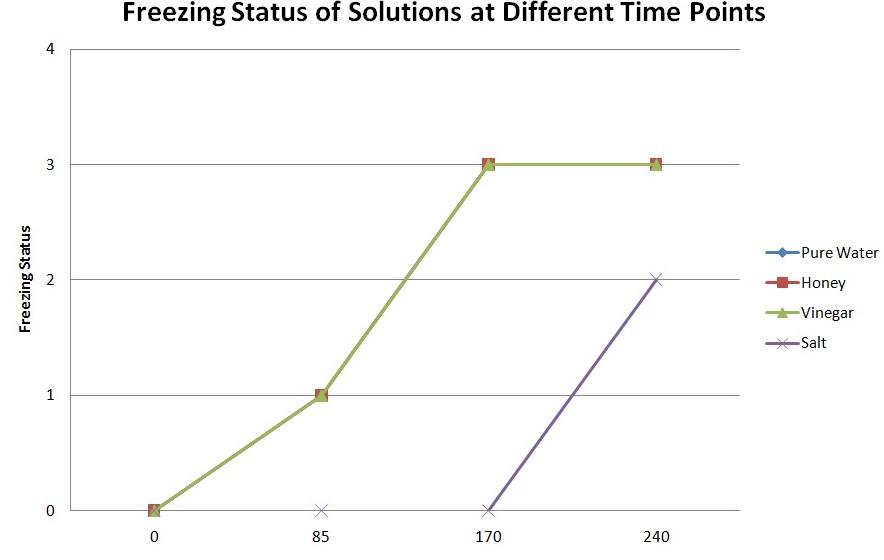
Ice started developing on each of the bowls (except for the one that contained the solution of salt) at around 85 minutes. The liquid in each of the bowls (except the one to which salt was added) was frozen solid after nearly 170 minutes have passed.
It took approximately 240 minutes for the bowl with the solution of salt to freeze, although it never became solid. In fact, it resembled slush. It was possible to stick the toothpick through it, whereas it was impossible with the other solutions.
The graph in Figure 1 below visualises the dependence of freezing status of water at different points of time:
Discussion and Conclusion
The hypothesis was partially correct; the water containing salt did not freeze solid at all, only turning to slush, which means the freezing point depression occurred in the salt solution. On the other hand, water in all the bowls except the one with the salt started freezing (almost) at the same time, and also was frozen solid almost at the same time. It is probable that the time when the solutions started freezing and became frozen solid was actually somewhat different, but the difference could not be detected because measurements were only taken each five minutes.
It may be possible to explain the different rates of freezing by the fact that the concentration of salt particles in salt solution was greater than the concentration of the particles of the other additives (vinegar and honey, for instance, already contained some water in them). Almost similar freezing rates of pure water, vinegar solution, and honey solution might also potentially be explained by differences in the heat capacity of these solutions.
All in all, the experiment yielded at least partially positive results. The salt solution took longer to freeze than the other solutions or the pure water, and in the end, was not frozen solid.
References
Bewick, S., Parsons, R., Forsythe, T., Robinson, S., & Dupon, J. (2017). 13.9: Freezing point depression and boiling point elevation: Making water freeze colder and boil hotter. Web.
Eaton, A. (2011). Chemistry: The effect of salt on the freezing point of water [Blog post]. Web.
Ge, X., & Wang, X. (2009a). A simple two-parameter correlation model for aqueous electrolyte solutions across a wide range of temperatures. Journal of Chemical & Engineering Data, 54(2), 179-186.
Ge, X., & Wang, X. (2009b). Estimation of freezing point depression, boiling point elevation, and vaporization enthalpies of electrolyte solutions. Industrial & Engineering Chemistry Research, 48(4), 2229-2235.