Introduction
All organisms play the role of hosts to one or more species of parasites, which can have a significant impact on their fitness. To respond to the threats from parasite infections, hosts learned to develop a range of behavioral, physiological, and morphological defense mechanisms that progressed to be effective in reducing the risk of infections or minimizing losses induced by parasites (Combes, 2001). These defense mechanisms allow organisms to either avoid encounters with a parasite (Moore, 2002), develop anti-parasitic characteristics (Hart, 2005) and immune responses (Frank, 2002), or compensate for the immune losses caused by parasites (Michalakis, 2009). The protective characteristics and properties hosts use to withstand the damage of parasites have been differentiated into two types: tolerance and resistance; therefore, this paper will focus on examining these traits and their significance to the field of immunopathology.
In the study of parasitology and disease ecology, and the ability of a host to tolerate or resist parasites has been given extra attention. First, it is important to differentiate between resistance and tolerance to apply these terms in further research. Biologists recognize that both terms refer to the ability of an organism to defend itself from harmful effects (Clarke, 1986); however, there is a difference in the way the defense mechanisms work. While resistance is the ultimate fitness of an organism to limit the burden of a parasite (Raberg, Graham, & Read, 2009), tolerance is the ability to limit the damage that may be caused by the parasite. This means that a host can be good at reducing the burden of a parasite but may not be the healthiest and thus unable to limit the damage caused by a parasite.
Resistance Vs Tolerance
Understanding the relationship between the terms of resistance and tolerance is essential to biologists that contemplate performing manipulations of host defenses through genetic manipulations or immune interventions. For instance, if there is a negative correlation between tolerance and resistance, interventions targeted at improving resistance can negatively impact the organism’s health. From the evolutionary and ecological perspective, the differentiation between tolerance and resistance lies in the fact that resistance protects the host’s health at the parasite’s expense while tolerance saves the host’s health from harm without any negative impact on the parasite (Raberg et al., 2009). Thus, it can be suggested that there is are some differences between the ecological and evolutionary consequences of tolerance and resistance:
I. According to Roy and Kirchner (2000), the evolution of resistance should minimize the occurrence of parasites in hosts while tolerance should have either a positive or neutral effect on the occurrence of parasites.
II. According to Woolhouse, Webster, Domingo, Charlesworth, and Levin (2002), because resistance negatively affects the fitness of a parasite, it can impose the selection on the parasite to overcome the defense of the host. This, in turn, may lead to imposing selection for improving host resistance, causing the antagonistic relationship between the parasite and its host (Ezenwa, Archie, & White, 2016). Contrary to this, tolerance cannot hurt the parasite’s performance; thus, there is no selection on the parasite for overcoming the host’s defense. Some authors (Rausher, 2001; Boots, 2008) put forward an argument that the evolution of tolerance will not result in the open-ended antagonistic coevolution. Therefore, because tolerance and resistance have radically opposite effects on infectious diseases and epidemiology, it is important not to confuse the two terms since it will contribute to a more in-depth understanding of the ecology and evolution of interactions between parasites and hosts.
Scientists that have been working with plants invested into examining the relative roles of tolerance and resistance in the process of withstanding against abiotic and biotic threats (Lefevre, Williams, & de Roode, 2011). To date, a large number of empirical studies characterized resistance as the ability of a plant to mitigate the severity of a parasite’s damage and tolerance was explained as the “slope of a reaction norm of fitness across a gradient of increasing damage” (Lefevre et al., 2011, p. 751). When it comes to parasitism, genotypes of plants that are highly resistant usually have lower infection rates as opposed to those that are less resistant. On the other hand, plant genotypes that are highly tolerant to parasites usually suffer smaller fitness reductions compared to those that are less tolerant (Lefevre et al., 2011).
The significance of these studies was not only in that they found that many organisms choose tolerance over resistance to defend themselves, but also in showing that the genetic variation intolerance is a widespread phenomenon (Koskela, Puustinen, Salonen, & Mutikainen, 2002). Because such type of polymorphism goes against the majority of theoretical predictions, many researchers have decided to investigate defense mechanisms capable of maintaining the genetic variation. In more recent years, theorists managed to develop models that point at the variation intolerance that can potentially evolve in cases when the mechanisms of tolerance maintain the fecundity (reproductive rate) of hosts rather than their survival (Best, White, & Boots, 2008). On the other hand, when hosting organisms proceed with tolerating an infection through controlling an even rate of their reproduction, the effect of parasites can be neutral, causing the variation of tolerance to evolve (Best et al., 2008).
Measuring Tolerance and Resistance
Exploring the phenomenon of tolerance to parasite organisms has become a crucial part of plant science, where researchers identified extensive variation in genes. In the context of evolution and ecology, tolerance results from the slope in the connection between a load of a parasite (x-axis in Figure 1) and the fitness of a host (y-axis in Figure 1).
During the last decade of the twentieth century, plant biologists were successful in developing a scientific framework used for measuring resistance and tolerance. Since then, it was found that there is a presence of heritable and environmentally induced variations of both tolerance and resistance (Stowe, Marquis, Hochwender, & Simms, 2000; Kover & Schaal, 2002). Plant scientists continued to study the processes of tolerance to determine new implications for breeding plants. Nevertheless, the advancements achieved by plant scientists have had practically no impact on the innovations in the studies of animal diseases. Immunologists and parasitologists have predominantly studied the ability of hosts to prevent the burdens caused by parasites (resistance) or the overall ability to maintain health when being under threat from parasites despite the severity of the burden (combined effects of resistance and tolerance) (Medina & North, 1998). These types of measures were usually used interchangeably in the scientific literature. However, only recently, the studies of animal diseases and immunology have integrated the empirical evidence used in plant literature (Restif & Koella, 2004).
In terms of measuring resistance and tolerance, a question arises: “can resistance and tolerance patterns be assessed through the use of an adaptive framework?” (Restif & Koella, 2004) Naturally, some scientists may hypothesize that the benefits of fitness and costs of resistance or tolerance can differentiate
Resistance
According to Simms and Triplett (1994), resistance is measured “as the inverse of infection intensity (number of parasites per host or unit host tissue); all else being equal, a lower intensity means an animal is more resistant” (p. 1979). Parasites included in the studies of resistance and tolerance usually vary from coevolved parasites (Sternberg et al., 2012), experimental infections (Vale & Little, 2012), natural (Blanchet et al., 2010), and less characterized (Vincent & Sharp, 2014). Methods of quantifying resistance can be used by the characteristics of a host-parasite system (Kutzer & Armitage, 2016), although such quantification always includes the measurement of infection intensity (parasite load).
For instance, if some researchers did not use experimental infection to quantify the resistance of a group of animals, others may use the self-selected group of animals that were exposed to a parasite, but were not infected to compare their overall fitness to those groups that were not exposed. Such a comparison can provide scientists with data about the cost of animals being exposed to parasites (Rohr, Raffel, & Hall, 2010). Resistance can also be examined through observational field studies, although they present other problems. For example, if there is no information about the dose of an infection and if a host does not have a lot of parasites, scientists cannot know if that particular host implemented a strategy of avoidance once the parasite was encountered or if the host has superior resistance compared to other representatives of the species (Graham et al., 2011).
A strategy of a host’s resistance can potentially have the following three implications (outcomes):
- A host gets rid of the parasite’s infection;
- Infection remains within the host at a stable rate;
- A host dies due to the infection (Kutzer & Armitage, 2016).
These outcomes can be associated with the measurement of a host’s fitness as well as other factors that pertain to the strategy of tolerance. For example, the survival of a host points at its fitness if it was expected that the infection would lead to the host’s death within the timeframe of the experiment. However, if the measure of fitness also includes fecundity, the death of the host will require researchers to select a sub-set of animals on which they will experiment further. Choosing the time at which the parasite should be analyzed is an important part of the research (e.g., a cross-sectional analysis will measure the host’s resistance at a specific point during the infection). On the other hand, variation in a host’s resistance can be falsely interpreted as tolerance if one group of the animals on which researchers experimented was faster in dealing with an infection than another. To deal with this issue, Raberg, Sim, and Read (2007) attempted to use the total number of parasite and their peak density, which gave the same results. Although, in the experiment with mice and malaria, there was a correlation between the total number of parasites and their density. It is important to note that a similar method to that of Raberg et al. (2007) may not give similar results within a different system of parasites and hosts. Other experimental studies examined different points in time when different phases (acute and chronic) of infection took place (Figure 1 below).
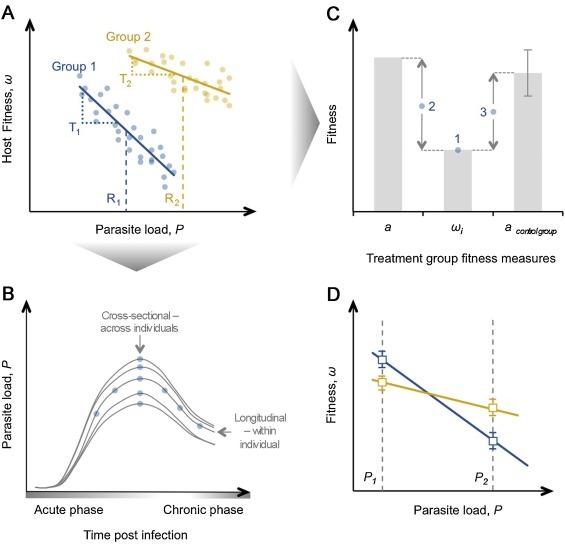
The studies concluded that different time points during an infection influence the emergence of different estimates of tolerance (Howick & Lazzaro, 2014). Although, if researchers conduct an observation field study, then it would be nearly impossible to acquire data about the specifics of every stage of infection due to the lack of information about the time point when the initial infection occurred. Another complication is associated with individuals showing a longitudinal variation in resistance, which is seen on individual trajectories of infection (Figure 1 above). Thus, obtaining information about the progression of the infection before analyzing resistance can be very useful to researchers working in this field.
Tolerance
Tolerance is measured as the regression slope of hosts’ resistance against the intensity of the disease: the lower the slope, the higher is the tolerance (Koskela et al., 2002). Thus, a statistically correct definition of tolerance should be that is the rate of changes in fitness along with the increase of parasite burden. Ecology and evolutionary biology define this trait as a reaction norm that describes how various groups of individuals respond to specific environmental conditions (Read, 1999). Following the explanation that tolerance is a slope, it can be stated that this trait (contrary to resistance) is impossible to measure by using one animal (Davies & Davies, 2010). Instead, resistance should be measured using a group of individuals from the same unit of hosts. Therefore, if one wishes to study the association between genetics and tolerance, it will be effective to measure the fitness of a genetically identical group of hosts that have a certain number of parasites in their systems. After measuring the fitness, it is important to compare the slopes among the different genetic units of that group (Matsumura, Arlinghaus, & Dieckmann, 2012). Genetic units may include breeds of domestic or laboratory animals or individuals that carry a specific allele at a specific locus (a gene’s position) (Raberg et al., 2009).
It is important to measure tolerance as a reaction norm because it this way, one can conclude that it is possible to observe fitness differences between types of hosts since they differ in their ability to withstand or limit the damage caused by the same parasite. If one is to measure tolerance in only one host, it may be impossible to conclude that variation between hosts originated from factors that are not associated with tolerance. To see the reason for it, let’s review the image (b) presented in Figure 2 below:
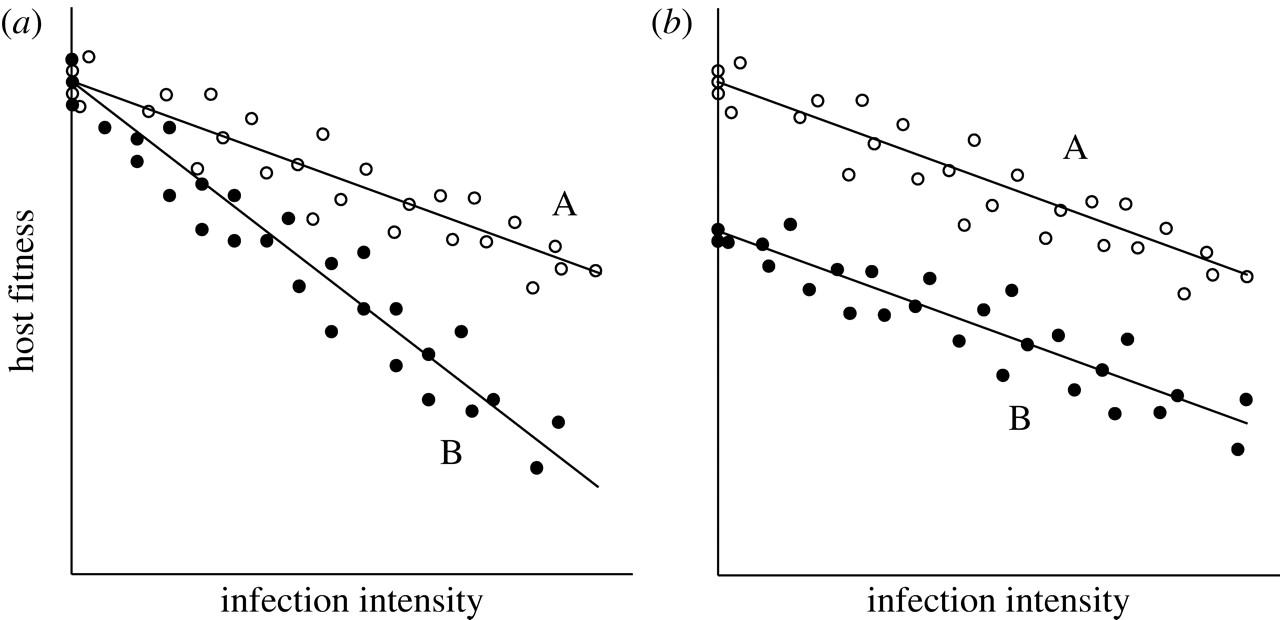
In the figure above, the types of hosts differ in their residual deviation from a common line of regression; however, the individual lines of regression are parallel to each other. Thus, no interaction can be seen between the burden of a parasite and the type of the host. Put differently, types of hosts can vary in intercept rather than slope (Raberg et al., 2009). Because there is a constant difference in fitness across different intensiveness of an infection, it may not be associated with the mechanisms that defend from parasites. On the other hand, the difference in fitness is likely caused by the variation in the traits of hosts (Stowe et al., 2000). As mentioned by Fry (1993), other traits of hosts were given the label of “general vigor” which relates directly to the concepts of “condition” and “resource acquisition” (p. 328). The last two concepts are predominantly used by scientists operating in the sphere of animal genetics and immunology.
To rule out a specific source or variation, scientists conduct tests that determine the statistical interaction between host genotype and the burden of a parasite. For example, let’s imagine that a scientist has an aim to study the tolerance of various breeds of cattle to a specific parasite, where the interest parameter is weight gain (a production trait). It is possible that different breeds can vary in the processes of weight gain despite regardless of a parasite’s presence. Therefore, the difference in weight gain when a parasite is present can occur owing to the natural difference. It will be ineffective to compare weight gain at a specific parasite load to find out how different cattle breeds respond and tolerate the parasite. A scientist needs to answer the following question in such cases: how does the difference in weight gain among different cattle breeds change in the presence of a parasite? If a significant statistical interaction between bread and load is found, then it can be concluded that the difference in weight gain changes with parasite burden – some breeds are worse at tolerating the parasite compared to others.
The approach of “reaction-norm” is also beneficial in the sense that it allows researchers to study the relationship between an organism’s fitness and the intensity of infection. There is no prior explanation of why such a relationship should be linear, as mentioned by Raberg et al. (2009). It is important to mention that if the relationship between fitness and the intensity of disease is non-linear, there may be drastic differences in fitness between types of hosts even in cases when there is a slight difference in the burden of a parasite (and vice versa) (Raberg et al., 2009). If to analyze the comparison of fitness and the intensity of an infection, one may get a false impression that types of hosts are different in their tolerance, while in reality they are placed at different positions along with a norm of common reactions (Kutcherov, 2016). By implementing the reaction norm approach, it will be possible to statistically control this potentially confusing factor (Henriksen, Dayton, Keyes, Carayon, & Hughes, n.d.).
In the majority of cases, biologists that studied host tolerance characterized it according to innate factors within different populations and genotypes; however, tolerance can also be a function of innate factors such as age or sex of the host. Therefore, it can be asserted that mechanisms that may influence host tolerance vary depending on the context. For example, in vertebrates, the inflammatory response to a parasite in conjunction with specific factors such as population or age can underpin the genetic characteristics connected to tolerance variation in natural systems of hosts-parasites (Medzhitov, Schneider, & Soares, 2012).
If to follow the logic, it can be asserted that tick, flea, and tapeworm parasitism may influence the fitness of a host; however, the study conducted by Jackson et al. (2014) suggested that there is a much more complex connection that governs the host’s fitness changes, shown on an example of infected field voles. The study found a dichotomous relationship between the immune strategy and the age of the vole; for example, a mature male showed a weaker resistance to macroparasite’s infection compared to immature representatives of the sample; however, they were more tolerant to the parasite (Jackson et al., 2014). Furthermore, older but more tolerant field voles experienced greater parasite intensity, so there was a positive correlation between body condition and a load of infection. In the study, GATA3 (a transcription factor related to Th2 immunity) (Kutzer & Armitage, 2016) expression was positively correlated with the load of the parasite, the condition of animals’ liver, and the condition of the body among mature voles. Among the population of mature voles, the macroparasites increased the expression of GATA3, resulting in the improved condition of their bodies but a decreased reproductive effort (Jackson et al., 2014). This research provided an example of costs associated with hosts’ tolerance.
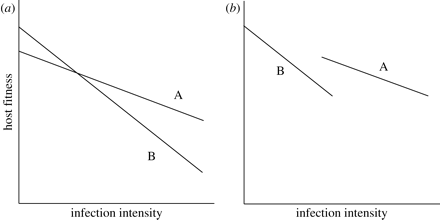
Constraining the evolution of tolerance within hosts also comes with costs and not only benefits. These costs show up in different contexts and different ways. First, high levels of a host’s tolerance can subsequently cause the reduction of fitness when parasites are absent. In the plant science, this processes is referred to as “allocation costs,” which are expected to increase because the maintenance of mechanisms for supporting tolerance requires some resources (Jackson et al., 2014); therefore, such resources should be taken from various traits that enhance the fitness of hosts. Allocation costs represent a negative correlation between a host’s tolerance and fitness in cases when parasites have been found in the organism of a host (an in Figure 3 below: a negative correlation between the intercept and the slope) (Jackson et al., 2014).
Several studies in the sphere of plant biology have determined that this type of cost exists within the context of tolerance to pathogens and parasites (Koskela et al., 2002). On the other hand, because the slope and the intercept parameters are dependent, plant scientists cannot reliably estimate the qualities of their connection.
Second, if a host is infected with a parasite, costs of tolerance may be presented in a form of a negative correlation between tolerance and resistance: less resistant genotypes are more tolerant and vice versa (Raberg et al., 2007) (b in Figure 3 above). According to Stowe et al. (2000), such a negative genetic correlation can be the consequence of either linkage disequilibrium (occurs when specific combinations of two traits are favored by correlational selection) between different loci or antagonistic pleiotropy (one gene can have opposite impacts on various traits).
Studies of tolerance and resistance in the context of animal parasites can benefit from a better understanding of genetic correlations between resistance and tolerance. The research conducted by Wambua et al. (2006) that focused on human malaria in Kenya found that people homo- or heterozygous for α+-thalassaemia exhibited a lower concentration of hemoglobin during the times without the infection compared to people homozygous for the wild-type. Raberg et al. (2007) also concluded that there was a negative genetic correlation between resistance and tolerance across different strains of mice. Therefore, the fact that is a relative number of experimental studies found the opposing effect of defense genes on host’s fitness suggests that such a correlation could be caused by antagonistic pleiotropy.
It is worth to point out the research conducted by Regoes et al. (2014) that aimed to determine whether there is a variation in HIV tolerance among humans. Because the progression of HIV is linked to the exhaustion of helper lymphocytes, rate of the disease’s progression is associated with the acute period of infection when a person first experiences symptoms similar to those of flu that occurs in response to the rapid processes in plasma (Regoes et al., 2014). The study concluded that the age at which an individual got an infection played a significant role in predicting the levels of tolerance. Furthermore, it was found that older individuals exhibited a more rapid pace of disease progression compared to the younger ones. Regions’ et al. (2014) study was the first research to identify the molecular basis of naturally segregating genetic variation for tolerance (Raberg, 2014). While the identification of genotype may have an impact on the sphere of immunopathology, the specific mechanisms for determining tolerance remain to be discovered in the future (Raberg, 2014).
Various approaches used to detail the effects of different factors on a host’s tolerance will help researchers find the underlying mechanisms or genetic characteristics linked to immune strategies. Moreover, such studies point to the importance of conducting a meaningful analysis of tolerance with regards to identifying hosts’ immunopathology along with the dynamics of parasite’s infection. It is unlikely that there is a mechanism that can predetermine a host’s tolerance; thus, it is expected that tolerance will vary as a function of several factors such as host’s age, sex, type of the parasite, infection intensity, immunocompetence, as well as other environmental factors that influence plasticity.
Empirical Evidence
Genetic Variation
It is considered that protozoans of the Plasmodium genus are the most well-researched parasites. They cause the disease of malaria in a vast number of animals. In their study, Raberg et al. (2007) attempted to apply a statistical framework to analyze tolerance to malaria in laboratory mice. The research used five different mice strains that had previously shown differences in resistance. To generate the variation in the intensity of infection (thus increase the statistical possibility to detect the genotype-to-burden interaction), mice were infected with one out of three clones of a parasite that differ in the severity of the infection they cause. Resistance to parasites was measured in the form of an inverse peak of parasite density. According to Mackinnon and Read (2004), the most regularly measured effects of parasite burden are anemia and weight loss, both of which are associated with animals’ mortality. Thus, Raberg et al. (2007) measured animals’ tolerance to malaria as a slope that showed a regression of anemia and weight loss against the highest density of a parasite. With regards to cases of both weight loss and anemia in mice, the regression slopes were different between strains of mice; this revealed a variation intolerance among them. Therefore, the genetic variation between different mouse strains has an impact on animals’ tolerance of the disease (Ayres & Schneider, 2008).
Environmental Variation
It can be stated that the presence of different infections simultaneously is a significant determinant of environmental variation of tolerance. Co-infecting parasites tend to affect each other in a variety of ways. According to Page, Scott, and Manabe (2006), immune-mediated interactions occur regularly and can independently impact the density of a parasite as well as the health of a host. For example, helminths often use suppressive responses to benefit their survival (Maizels et al., 2004). However, at the same time, they respond to other threats from infections by using the so-called “bystander effects” (Kamal & Khalifa, 2006). Helminths are organisms that can reduce the resistance of animals (e.g., mice) to parasites.
More importantly, an animal’s immune response to the co-infection of helminths can either increase or decrease the severity of the disease induced by a parasite without altering its density (Furze, Hussel, & Selkirk, 2006). Thus, it can be concluded that a co-infection of helminths can reduce animals’ tolerance to other parasites. Moreover, in some instances, hosts co-infected with a helminth may be at once tolerant and less resistant to other infections. For instance, helminths co-infected hosts can be less resistant to high densities of malaria but be more tolerant of immunopathological symptoms of cerebral malaria (Specht & Hoerauf, 2007). To conclude, it may be important to decide whether in the future it will be worth implementing medical interventions for promoting resistance or tolerance in animal populations infected by different parasites simultaneously (Geerts & Gryseels, 2000).
Genetic Knockouts
Some of the most valid evidence that animal organisms can defend themselves from infections not only with the help of natural resistance but also tolerance that was developed from experiments associated with genetic engineering (Whitelaw & Sang, 2005). For instance, scientists developed a species of ‘knockout mice’ in that the elimination of a specific gene lead to the altered severity of a disease without any changes in the intensity of parasites (Raberg et al., 2009). Even though such findings do not point to natural genetic variation, they show that animals can have disease control mechanisms that do more than just reduce the burden of parasites. This also points to the biological mechanisms that may reinforce an organism’s tolerance to parasites (Goldberg & Marrafini, 2015).
Studies of knockouts have also found the existing mechanisms of tolerance in invertebrates. In their study “Identification of Drosophila mutants altering defense to and endurance of Listeria monocytogenes infection”, Ayres, Freitag, and Schneider (2008) successfully infected a thousand mutant lines of D. melanogaster (species of fly) with a bacterial pathogen Listeria monocytogenes. The study showed that 18 mutants were much more to dying from infection compared to wild types of flies. In 12 mutants, the intensity of infection elevated, which suggested that the mutants had inadequate pathways of resistance. The remaining 6 mutants were much more prone to dying from infection in cases when elevated pathogen titers were absent. Therefore, these mutants were likely to defect in the tolerance of pathways (Raberg et al., 2009). It is predicted that the tools available to scientists studying fly genetics will reveal the nature and the function of these pathways (Alberts, 2002).
Issues Related to the Evolution of Tolerance and Resistance
A popular question with regards to the evolutionary dynamics of tolerance is related to the issue of tolerance and coevolution (Raberg et al., 2009). Therefore, one of the most curious implications of tolerance is that it is much more likely to fixate as a defense mechanism rather than a resistance mechanism (Roy & Kirchner, 2000). This occurs because mechanisms of resistance usually work by eliminating parasites that favored them from the very beginning (Raberg et al., 2009). Consequently, as specific mechanisms of resistance near fixation in a certain population, parasites should change or rot off, leaving the resistance mechanism useless or unnecessary. Contrary to this, tolerance cannot minimize the selective pressure that favored parasites. Thus, it can fixate much more easily (Combes, 2000). Such insight points to an interesting possibility: are the majority of defense mechanisms occurring during evolution are equal to tolerance mechanisms (Baucom & de Roode, 2010)? It can be assumed that hosts do not get sicker from an infection because of the long succession of tolerance mechanisms that fixate in populations of organisms (Lloyd-Smith, Schreiber, Kopp, & Getz, 2005; Matthews et al., 2005).
While it has already been argued that the reaction norm approach is the most effective for demonstration tolerance variation, this approach can lead to some factors that may cause spurious tolerance variation (Raberg et al., 2009). For the sake of discussion, it can be assumed that the experimental aim of the approach is to elicit the genetic variation intolerance, and therefore, the comparison of tolerance can be conducted across the genotypes of hosts (Raberg et al., 2009). The problems are the same when tolerance is being compared across other types of hosts, for example, hosts of different gender or ages, or hosts being raised in different conditions (Raberg et al., 2009).
For scientists to deal with the problem, they should start with measuring parasite burden in a manner that would reflect a host’s resistance. If this is not done, then there will be difficulties with making reliable differentiation between the qualities of resistance and tolerance. In the majority of experimental studies, researchers use the measure of parasite burden, which is the density of infection at its peak, as mentioned by Raberg et al. (2017). Nevertheless, in cases when scientists measure a host’s ability to deal with the density of parasites, they rarely take into consideration all characteristics of the organism that help hosts to be resistant. It is crucial to mention that in cases of variation between the clearance rate of infection, biologists often measured peak density, which is a tremendous mistake because in such scenarios resistance of hosts can be misidentified as tolerance (Raberg et al., 2009). This issue can be eliminated by finding an appropriate summary measure of infection burden within the time during which a parasite is present in a host’s organism (Raberg et al., 2009). For example, this measure can be the average density of parasites or the accumulative number of parasites accumulated in a host’s organisms up to the point when the fitness or the severity of the disease is measured (Raberg et al., 2009).
The second point in eliminating the issue about the reaction norm approach is testing whether reaction norms are linear or nonlinear (Raberg et al., 2009). If it is found that reaction norms are nonlinear, then the following issues arise:
1. With regards to nonlinear reaction norms, a statistical relationship between the genotype of a host and the linear term in regression points at the genetic tolerance variation. Furthermore, tolerance will be presented as a linear coefficient in the case if the relationship between the linear term and the genotype is connected to the linear term of a disease’s intensity. However, if the relationship includes a quadratic term, it is more complicated to determine the tolerance of a host genotype (as seen in Figure 4 (a) below). A solution to this may be to scale reaction norms for various genotypes of hosts for them to have the same intercept, and then use the “under the curve” are as a tolerance measure (Raberg et al., 2009).
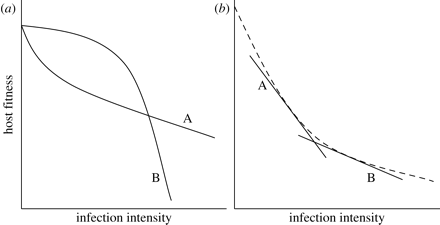
2. If reaction norm is nonlinear, then the average density of infection varies among host genotypes (may occur in cases of difference in genotypes’ resistance), integrating a linear term in the regression may facilitate the production of a falsely significant interaction as well as a spurious correlation between resistance and tolerance (as seen in (b) Figure 4 above) (Raberg et al., 2009). For scientists to eliminate this potential issue, they should create a relatively large overlap in parasite burden within different host genotypes (Raberg et al., 2009). For instance, in the studies of herbivores, such overlap can be achieved through an experimental imposition of fixed damage levels (Tiffin & Inouye, 2000). Nevertheless, maintaining fixed intensities of disease is often impossible when dealing with parasites since the mildness or the severity depends on the relationships between hosts and parasites (Tiffin & Inouye, 2000). Interestingly, scientists can influence the variation of infection intensity through the use of parasite clones with various rates of intensity (Raberg et al., 2009).
The third point is that when the intensity of infection cannot be controlled experimentally at specific fixed levels, there is a risk of concluding that the estimation of tolerance is biased by an unmeasured factor that influences the fitness of hosts and the intensity of infection independently (Raberg et al., 2009). If these factors continue to affect all genotypes of hosts in the same manner, their impact will not have an impact on the genetic variation of tolerance. Although, it can wind up the estimation of genetic variation for tolerance with the presence of a genotype-by-environment interaction (Tiffin & Inouye, 2000). This can become a problem for researchers when there the magnitude of such an interaction is large about the effect of the infection on fitness; in other cases, the effect on tolerance will be small (Tiffin & Inouye, 2000). It is important to mention that this particular problem did not receive enough attention; therefore, further research is needed before it is possible to determine how relevant it is to the study of parasitology. For now, researchers suggest the following methods to minimize the potential problem:
- Conduct research inhomogeneous environments to limit the interaction between environmental variables and host genotypes;
- Experimentally enhance the variation in the intensity of infection.
It can be concluded that studies on tolerance should receive attention beyond the sphere of evolutionary ecology, as pointed out by researchers (Rausher, 2001; Schafer, 1971). When speaking about the context of plant diseases, the possibility of various co-evolutionary outcomes is encouraged by resistance and tolerance raising the prospect of various manipulations of host defenses that may be either proven or disproven by evolution (Horns & Hood, 2012). By not imposing the process of selection for countermeasures of pathogens, animal or public health interventions targeted at increasing tolerance may be held back by the pathogen evolution compared to those interventions that aim to boost organisms’ resistance (Mackinnon, Gandon, & Read 2008).
When exploring resistance and tolerance of organisms in the context of the agricultural sector, the attempts to make a selection for the increased yield under the threat of parasites can result in nothing (Poulin, 2010). The final issue regarding disease epidemiology is associated with the existence of “super-spreaders” – hosts responsible for developing large numbers of secondary parasite transition cases (Stein, 2011). Thus, identifying and eliminating super-spreaders has a potentially significant impact on the disease incidence rate (Blumberg, Funk, & Pulliam, 2014). About this, the following question for future research arises: are super-spreaders tolerant to parasites hosts in populations that are more resistant than others? (Rohr, Raffel, & Hall, 2010).
Conclusion
To conclude, resistance and tolerance are components of the host’s defense against parasites that differ in the way the mechanisms of defense operate. Together, the two components can give scientists an idea of how well hosts are protecting themselves from the adverse effects of parasitism processes. Distinguishing between tolerance and resistance is important for understanding that hosts that are the best at protecting themselves from the effects of parasitism are not necessarily the healthiest. Furthermore, the paper showed genetic implications for developing tolerance to parasites.
The studies of tolerance and resistance in the context of plant and animal ecology have been becoming more popular due to the possibility of conducting experiments within different contexts. It has been found that tolerance can vary over the lifespan of infection, at different points of a host’s lifespan, as well as in response to various environmental factors. The same way in which hosts differ in resistance, tolerance variation is a universal trait since the bulk of published literature found support for it in some form. Tolerance variation is an interesting topic that should be further explored because some theoretical models usually predicted it to be fixed to a specific population of hosts. Examining tolerance and resistance has proven to be useful for determining how hosts can be protected against possible threats of infection as well as what procedures can be undertaken to enhance their ability to withstand the burden of parasite infection and overcome the disease.
On the other hand, resistance and tolerance have their limitations. For instance, there could be a weak connection between a host’s fitness and parasite load (as found in the study by Regoes et al., 2014). Given the complex nature of intra-specific interactions between hosts and parasites, the weak connection between parasite load and host fitness is not surprising. Thus, researchers are presented with a challenge to explain the sources of variance among the reaction norm of tolerance. Nevertheless, tolerance- and resistance-centered studies have the potential to provide insightful information about the interactions between hosts and parasites to enhance the knowledge in the field of immunology and offer effective solutions to eliminating the previously identified problems.
References
Acute and chronic phases of infection [Image] (2016). Web.
Alberts, B. (2002). Studying gene expression and function. Web.
Ayres, J., & Schneider, D. (2008). Two ways to survive an infection: What resistance and tolerance can teach us about treatments for infectious diseases. Nature reviews. Immunology, 8(11), 889-895.
Baucom, R., & de Roode, J. (2010). Ecological immunology and tolerance in plants and animals. Functional Ecology, 25(1), 18-28.
Best, A., White, A. & Boots, M. (2008). Maintenance of host variation in tolerance to pathogens and parasites. Proc Natl Acad Sci USA, 105(20), 786-791.
Blumberg, S., Funk, S., & Pulliam, J. (2014). Detecting differential transmissibility that affect the size of self-limited outbreaks. Web.
Boots, M. (2008). Fight or learn to live with the consequences? Trends in Ecology and Evolution, 23(6), 248-250.
Clarke, D. (1986). Tolerance of parasites and disease in plants and its significance in host-parasite interactions. Adv. Plant Pathol, 5, 161-197.
Combes, C. (2000). Selective pressure in host-parasite systems. J Soc Biol, 194(1), 19-23.
Combes, C. (2001). Parasitism: The ecology and evolution of intimate interactions. Chicago, IL: University of Chicago Press.
Davies, J., & Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews, 74(3), 417-433.
Ezenwa, V., Archie, E., & White, L. (2016). Host behavior-parasite feedback: An essential link between animal behavior and disease ecology. Proceedings of the Royal Society B: Biological Sciences, 283, 1828-1835.
Frank, S. (2002). Immunology and evolution of infectious disease. Princeton, NJ: Princeton University Press.
Fry, J. (1993). The general vigor problem – Can antagonistic pleiotropy be detected when genetic covariance are positive? Evolution, 47(10), 327-333.
Furze, R., Hussell, T., & Selkirk, M. (2006). Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infection and Immunity, 74(10), 1924-1932.
Geerts, S., & Gryseels, B. (2000). Drug resistance in human helminths: Current situation and lessons from livestock. Clinical Microbiology Reviews, 13(2), 207-222.
Goldberg, G., & Marraffini, L. (2015). Resistance and tolerance to foreign elements by prokaryotic immune systems – Curating the genome. Nature Reviews Immunology, 15, 717-724.
Graham, A., Shuker, D. Pollitt, L., Auld, S., Wilson, A., & Little T. (2011). Fitness consequences of immune responses: strengthening the empirical framework for ecoimmunology. Functional. Ecology, 25(1), 5-17.
Hart, B. (2005). The evolution of herbal medicine: behavioral perspectives. Anim Behav, 70, 975-989.
Henriksen, K., Dayton, E., Keyes, M., Carayon, P., & Hughes, R. (n.d.). Chapter 5: Understanding adverse events: A human factors framework. Web.
Horns, F., & Hood, M. (2012). The evolution of disease resistance and tolerance in spatially structured populations. Ecology and Evolution, 2(7), 1705-1711.
Howick, V., & Lazzaro, B. (2014). Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol Biol, 14, 56-57.
Intensity of infection [Image]. (2009). Web.
Kamal, S., & Khalifa, K. (2006). Immune modulation by helminthic infections: worms and viral infections. Parasite Immunology, 28(17), 483-496.
Koskela, T., Puustinen, S., Salonen, V. & Mutikainen, P. (2002). Resistance and tolerance in a host plant–holophrastic plant interaction: Genetic variation and costs. Evolution, 56, 899-908.
Kover, P., & Schaal, B. (2002). Genetic variation for disease resistance and tolerance among Arabidopsis thaliana accessions. PNAS, 99(2), 11 270-11 274.
Kutcherov, D. (2016). Thermal reaction norms can surmount evolutionary constraints: Comparative evidence across leaf beetle species. Ecology and Evolution, 6(14), 4670-4683.
Lefevre, T., Williams, A., & de Roode, J. (2011). Genetic variation in resistance, but not tolerance, to a protozoan parasite in the monarch butterfly. Proceedings of the Royal Society, 278, 751-759.
Lloyd-Smith, J., Schreiber, S., Kopp, P., & Getz, W. (2005). Super-spreading and the effect of individual variation on disease emergence. Nature, 438(12), 355-359.
Mackinnon, M., Gandon, S., & Read, A. (2008). Virulence evolution in response to vaccination: The case of malaria. Vaccine, 26(48), 42-52.
Maizels, R., Balic, A., Gomez-Escobar, N., Nair, M., Taylor, M., & Allen, J. (2004). Helminth parasites – Masters of regulation. Immunological Reviews, 201(11), 89-116.
Matsumura, S., Arlinghaus, R., & Dieckmann, U. (2012). Standardizing selection strengths to study selection in the wild: A critical comparison and suggestions for the future. BioScience, 62(12), 1039-1054.
Matthews, L., Low, J., Gally, D., Pearce, M., Mellor, D., Heesterbeek, J.,…Woolhouse, M. (2005). Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. PNAS, 103(3), 547-552.
Medina, E., & North, R. (1998) Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology, 93(10), 270-274.
Medzhitov, R., Schneider, D., & Soares, M. (2012). Disease tolerance as a defense strategy. Science, 335, 936-941.
Michalakis, Y. (2009). Parasitism and the evolution of life history traits. In F. Thomas, J. F. Gue´gan & F. Renaud (Eds.), Ecology and evolution of parasitism (pp. 11-36). Oxford, UK: Oxford University Press.
Moore, J. (2002). Parasites and the behavior of animals. Oxford, UK: Oxford University Press.
Page, K., Scott, A., & Manabe, Y. (2006). The expanding realm of heterologous immunity: Friend or foe? Cellular Microbiology, 8(10), 185-196.
Poulin, R. (2010). Parasite manipulation of host behavior: An update and frequently asked questions. In J. Brockmann (Ed.), Advances in the study of Behavior (pp. 151-186). Burlington, Academic Press.
Raberg, L. (2014). How to live with the enemy: Understanding tolerance to parasites. PLOS Biology, 12(11), 1-4.
Raberg, L., Graham, A., & Read, A. (2009). Decomposing health: Tolerance and resistance to parasites in animals. Biological Sciences, 364(1513), 37-49.
Raberg, L., Sim, D., & Read, A. (2007). Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science, 318(16), 812-814.
Rausher, M. (2001) Co-evolution and plant resistance to natural enemies. Nature, 411 (2), 857-864.
Reaction norms of different host genotypes [Image]. (n.d.). Web.
Read, A. (1999). What can evolutionary biology contribute to understanding virulence? In S. Stearns (Ed.), Evolution in health and disease (pp. 205-215). Oxford, UK: Oxford University Press.
Regoes, R., McLaren, P., Battegay, M., Bernasconi, E., Calmy, A., Günthard, H… Fellay, J. (2014). Disentangling human tolerance and resistance against HIV. PLoS Biol, 12, 1-10.
Restif, O., & Koella J. (2004). Concurrent evolution of resistance and tolerance to pathogens. The American Naturalist, 164(7), 90-102.
Rohr, J., Raffel, T., & Hall, C. (2010). Developmental variation in resistance and tolerance in a multi-host-parasite system. Functional Ecology, 24(5), 1110-1121.
Roy, B. A, & Kirchner, J.W. (2000). Evolutionary dynamics of pathogen resistance and tolerance. Evolution, 54, 51-63.
Simms E., & Triplett, J. (1994). Costs and benefits of plant responses to disease: resistance and tolerance. Evolution, 48(3),1973-1985.
Specht, S., & Hoerauf, A. (2007). Does helminth elimination promote or prevent malaria? The Lancet, 369(10), 446-447.
Stein, R. (2011). Super-spreaders in infectious diseases. International Journal of Infectious Diseases, 15(8), 510-513.
Sternberg, T., Lefèvre, J., Li, C., de Castillejo, H., Li, M., Hunter, J., & de Roode, C. (2012). Food plant derived disease tolerance and resistance in a natural butterfly-plant-parasite interaction. Evolution, 66, 3367-3376.
Stowe, K., Marquis, R., Hochwender, C., & Simms, E. (2000). The evolutionary ecology of tolerance to consumer damage. Annual Review of Ecological Systems, 31(5), 565-595.
Tiffin, P., & Inouye, B. (2000). Measuring tolerance to herbivory: accuracy and precision of estimates made using natural versus imposed damage. Evolution, 54, 1024-1029.
Types of tolerance [Image]. (n.d.). Web.
Vale, P., &. Little, T. (2012). Fecundity compensation and tolerance to a sterilizing pathogen in Daphnia. J Evol Biol, 25, 1888-1896.
Whitelaw, C., & Sang, H. (2005). Disease-resistant genetically modified animals. Review of Science Technology, 24(1), 275-283.
Woolhouse M., Webster, J., Domingo, E., Charlesworth, B., & Levin, B. (2002). Biological and biomedical implications of the co-evolution of pathogens and their hosts. Natural Genetics, 32(14), 569-577.