Latent heat is the energy that is accumulated or released in a specific thermodynamic system as a result of a constant temperature process (Lutgens and Tarbuck 375). The aforementioned process can be observed in the course of a change of state of matter and the following release or accumulation of energy.
In contrast to melting, which requires that a substance should change its states in an orderly fashion (e.g., from liquid to gaseous, from solid to liquid, etc.), sublimation presupposes switching from solid to gas and vice versa without going through the liquid phase. While melting occurs under normal conditions, subversion requires a significant change in pressure (Lutgens and Tarbuck 381).
Since in cool air, the particles have a low kinetic energy and move slower, it will take less water to saturate it, whereas the hot air will hold more water (Lutgens and Tarbuck 382).
The relative humidity of air can be changed by changing either the temperature of the thermodynamic system in question or the pressure in the system under consideration. Afterwards, the relative humidity rates will either drop or rise (Lutgens and Tarbuck 388).
A dew point is the combination of temperature and pressure, at which water starts to vapor and the vapor turns into water (Lutgens and Tarbuck 389).
The phenomenon of a glass sweating can be explained by the fact that, due to a comparatively high thermal conductivity of the glass, the air around it will become quite cool as well. Since cool air holds less water than the hot one, the water vapor in the air will condense into droplets and sink on the outside of the glass. As a result, the beads of “sweat” appear on the glass (Lutgens and Tarbuck 393).
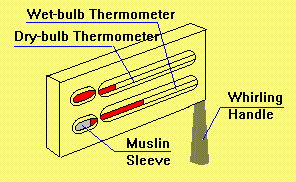
A sling psychrometer, or a hygrometer, consists of two thermometers, which are supposed to measure both the air temperature and the so-called “wet-bulb” temperature; thus, the dew point can be calculated, which, in its turn, will help define the relative humidity rates (Lutgens and Tarbuck 396).
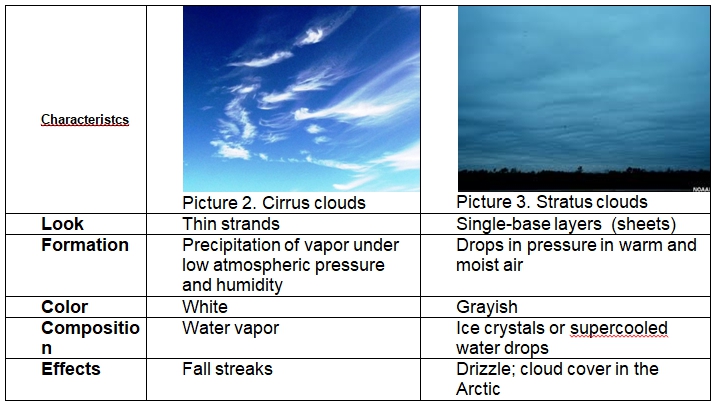
There are several ways to distinguish between cirrus and stratus clouds:
Fog is traditionally identified as small particles of H2O either in its liquid (water drops) or solid (ice crystals) state suspended in the atmosphere (Lutgens and Tarbuck 381). Typically, fog is confused with mist; the latter, however, is usually less thick and allows for a better visibility.
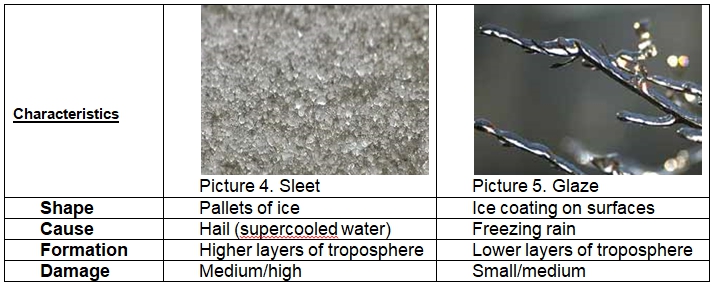
Though sleet and glaze might look similar and be composed of the same element, there is a range of tangible differences between them (Precipitation – Millersville University: Meteorology 1–5):
Hail stone, also known as sleet, forms from large drops of water in strong updrafts. As cool air descends and warm air rises, supercooled water starts turning to ice pellets, which results in hail (Lutgens and Tarbuck 402).
Works Cited
Lutgens, Frederick K. and Edward J.Tarbuck. “Heating the Atmosphere.” Foundations of Earth Science. 7th ed. Upper Saddle River, NJ: Prentice Hall. 2014. 373–409. Print.