The issues covered in this week’s discussion will include the pathophysiology of Alzheimer’s disease (AD), particularly, the tools that junior and senior students can use to educate patients on the subject matter. By the end of the session, the audience will be capable of:
- Providing the target population with the essential information about the identification of AD symptoms;
- Educating patients about pathophysiology with the help of visual aids;
- Teaching patients to recognize the symptoms and choose the appropriate resources for managing the problem;
- Understanding the role of media in learning new information about AD and its treatment options.
Handout
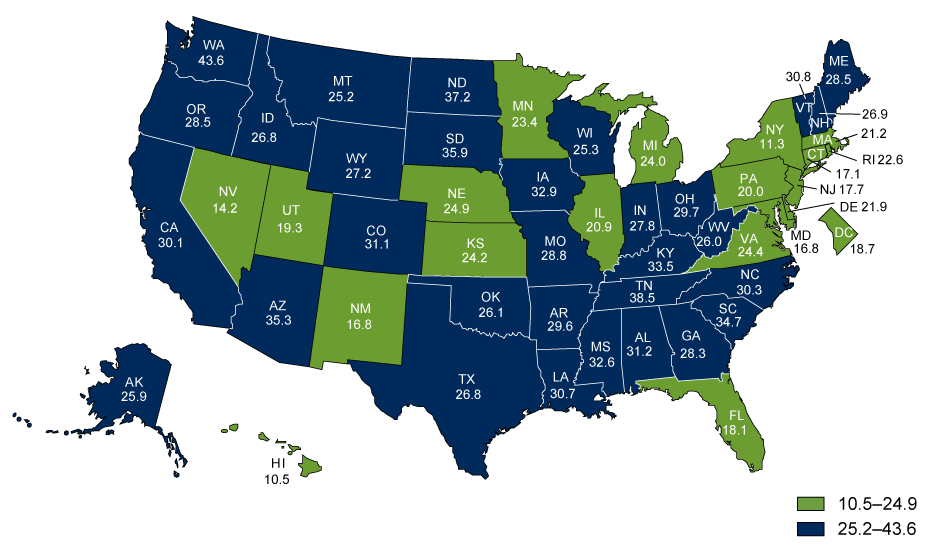
Alzheimer’s disease is a type of multifarious neurodegenerative disease that progresses consistently as the patient experiences neuronal loss (Norton, Matthews, Barnes, Yaffe, & Brayne, 2014). According to the Alzheimer’s Association (2016), 13.8 billion people in the U.S. suffer from the disorder. Furthermore, the death toll has been increasingly high, as shown in Figure 1. The disease occurs at both intra- and extracellular levels. The former is characterized by the development of the neurofibrillary tangles, whereas the latter implies the development of amyloidal protein deposits. Each of the phenomena triggers the further creation of senile plaques (Norton et al., 2014).
There are currently several hypotheses about the pathophysiology of AD. However, one of the hypotheses is viewed as more credible than others. Defined as the Αβ hypothesis, the identified supposition implies that Αβ oligomers trigger the development of synaptic impairment and, therefore, affect the functioning of the brain (McCance, Huether, Brashers, & Rote, 2014). Amyloid plaques, in turn, are developed at the later stages of the AD progression, as the theory states.
The theory implies that the amyloid precursor protein (APP) should be split by ∝- secretase, whereas the processing thereof should be carried out with the help of the β- and γ-secretase. Thus, Αβ peptide production is enhanced, and the amount thereof exceeds the norm. Consequently, soluble oligomers are formed, causing the appearance of the beta-sheet conformation. The results of the identified process can be deemed as detrimental to the neurons of the human brain and include the following possible outcomes: oxidative damage, tau hyperphosphorylation, as well as synapses and mitochondria intoxication. As a result, the rapid process of brain degeneration is launched immediately as neurons start disintegrating, as shown in Figure 2 (Kumar & Ekavali, 2014).
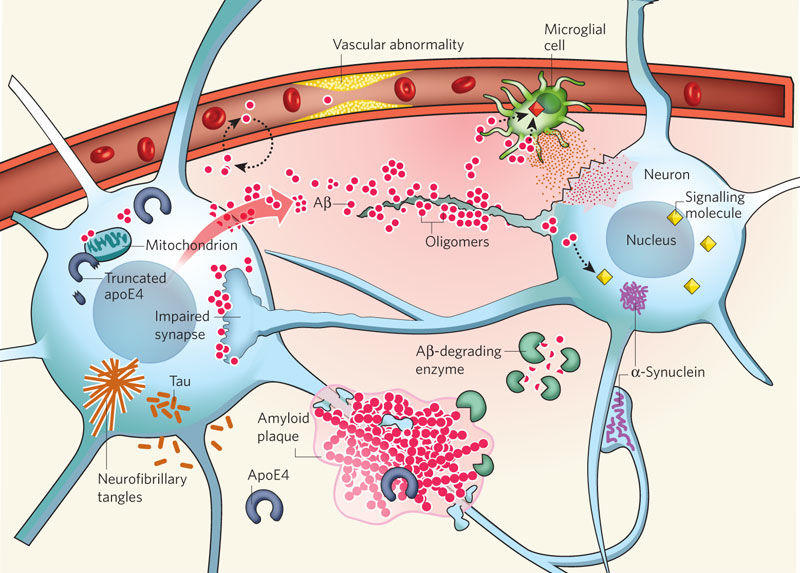
Therefore, the accumulation of Αβ plaques is currently viewed as the primary factor causing the development of AD in patients. It should be noted, though, that the recent researches of chronic brain injury and ischemia show that Aβ should be viewed as a nonspecific marker of brain damage (McCance et al., 2014). As shown in Figure 1, the Aβ depositions are not linked specifically to the issue of AD. Therefore, the development of neurofibrillary tangles in the patient’s brain can be deemed as the area that needs further studies.
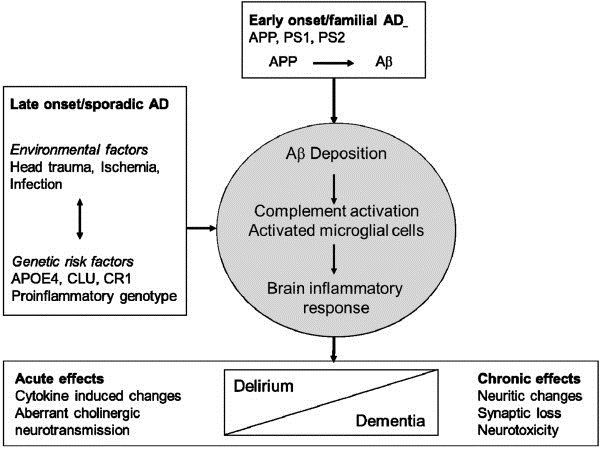
That being said, there is a range of evidence supporting the identified theory. For instance, a recent study showed that APP ectodomain serves as a ligand for the TNFRSF21 protein, also known as the Death Receptor 6 (DR6) (). In other words, deprived of the opportunity to grow, APP is caused to develop a cleavage that contributes to the release of the ectodomain that produces caspases 3 and 6, therefore, leading to the production of the DR6 protein. Consequently, the plaques are produced, and dementia (AD) progresses, as shown in Figure 3 above.
Evaluation of AD
To determine the development of AD, a nurse must examine the patient’s medical history thoroughly. Furthermore, the tests of the patient’s mental status and mood will have to be carried out, followed by a medical and physiological examination.
Treatment
The treatment options include cholinesterase inhibitors of memory loss and interventions aimed at reducing anxiety and depression. The management of changes in patients’ sleep patterns is also encouraged.
Video Summary
The Osmosis channel (2016) offers a very useful video that can shed a lot of light on the pathophysiology of AD. In their video, the owners of the Osmosis channel (2016) explain the concept of Alzheimer’s disease in great detail, yet rather simply. The narrator details how beta-amyloid plaque is collected in the human brain and, therefore, leads to neurofibrillary tangles in the brain. As a result, the atrophy of the brain begins, followed by symptoms such as memory deterioration. The video can be used as a perfect example of how the pathophysiology of Alzheimer’s disease can be explained to patients, thus, cultivating awareness among the target population. When coupled with visual aids, the said lecture helps get a deep insight into the nature of the disease and its pathophysiology.
Collapse
- Alzheimer’s Disease. AD is rapidly progressing dementia that is caused by a split of APP and the further development of amyloid plaques in the patient’s brain.
- Signs and symptoms. Memory loss, problem-solving skills deterioration, inability to perform routine actions.
- Pathophysiology. Development of beta-amyloid plaques in the patient’s brain.
- Evaluation. Study of the patient’s medical records, physiological and psychological examination.
Terms
- Neurofibrillary tangles – tau protein aggregates.
- Amyloidal protein deposits – teh aggregates triggering the structure entanglements.
References
Alzheimer’s Association. (2016). 2016 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12(4), 459-509.
Eikelenboom, P., Hoozemans, J. J. M., Veerhuis. R., Exel, E. V., Rozemuller. A. J. M., & Gool, W. A. V. (2012). Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer’s disease. Research & Therapy, 4(1), 15-23.
Kumar, A., & Ekavali, A. S. (2014). A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacological Reports, 67(2),195-203.
McCance, K. L., Huether, S. E., Brashers, V. L., & Rote, N. (2014). Pathophysiology: The biologic basis for disease in adults and children (7th ed.). Maryland Heights, MO: Mosby Elsevier.
Norton, S., Matthews, F. E., Barnes, D., Yaffe, K., & Brayne, C. (2014). Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurology, 13(8), 788-794.
Osmosis channel. (2016). Alzheimer’s disease – Plaques, tangles, causes, symptoms & pathology. YouTube.
Sindi, S., Mangialasche, F., & Kivipelto, M. (2015). Advances in the prevention of Alzheimer’s disease. F1000Prime Reports, 7(1), 50-61. doi:10.12703/P7-50.