Introduction
Flagyl ER is one of the common medications that help prevent the symptoms of bacterial and parasite infections. It is a brand of metronidazole that has been widely successful in treating infections in various body parts, including skin, joints, respiratory tract, stomach, and reproductive organs. Ultimately, the current project paper thoroughly examines Flagyl ER and describes its class, purposes, uses, dosages, and other pharmacological characteristics.
Class and DEA
Flagyl-ER (metronidazole) is an antibiotic with highly efficient antibacterial/antiprotozoal qualities. It belongs to the nitroimidazole class, which focuses specifically on bacterial and parasite infections (Memon, 2021). Antibiotics of this group alter the DNA of the affected bacteria, gradually leading to its death and mitigating the condition (Memon, 2021). They are particularly effective for treating skin, gastrointestinal, respiratory, and vaginosis bacterial infections, which are the primary target areas of Flagyl-ER (Memon, 2021). Considering the DEA class, Flagyl-ER is a prescribed medication, and it might cause multiple side effects (“Metronidazole – Drug,” n.d.).
They include fever, seizures, nausea, headaches, sleep disorders, and other problems, depending on the patient’s pre-existing medical conditions and drug resistance (“Flagyl ER,” 2021). Moreover, its application has been found to cause carcinogen activity in mice, implying that humans might suffer from a similar effect to a lesser degree (“Metronidazole – Drug,” n.d.). All precautions clearly designate that Flagyl-ER should be used only when necessary, and its DEA class is justified.
Flagyl ER Description and Brand Names
Consequently, it is crucial to describe the drug to understand its purposes and functions. As mentioned briefly before, Flagyl ER is an antibiotic of the nitroimidazole class, meaning that it is most effective for treating bacterial, parasitic, and protozoal infections. The active component of Flagyl ER is metronidazole, and its chemical formula is presented below in Figure 1:
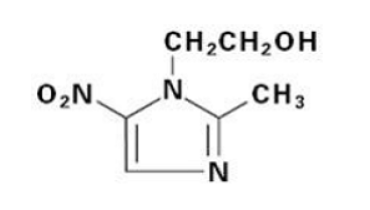
The application methods of metronidazole are oral, vaginal, topical, and parenteral, while Flagyl ER is specifically an oral medication (“Flagyl ER,” 2021). In addition to Flagyl ER, there are multiple brand names that treat bacterial infections and have metronidazole as an active component. Some of the most prominent drugs include Flagyl, MetroCream, Noritate, Rosadan, NUVESSA, Vitazol, Vandozole, and Nydamax (“Metronidazole – Drug,” n.d.). The differences between brands typically concern the method of application and the bottle/gel size.
How Supplied and Dosages
Despite the various supply methods of metronidazole, Flagyl ER is only available in oral tablets. The common size is 750mg, which is readily available in most pharmacies (“Metronidazole – Drug,” n.d.). Although one course for most treatments with Flagyl ER should not exceed one week, the 750mg package contains thirty tablets (“Flagyl ER,” 2021). Since patients should only intake one tablet per day and for no longer than seven consecutive days, the package can last for several courses. Lastly, users should store Flagyl ER in dry places in the temperature range from 59F to 86F.
The dosage is the most significant area of concern because Flagyl ER is associated with multiple side effects and potential risks of carcinogen activity. Depending on the target area, the dosage might slightly differ; however, the threats of the active component are imminent, and patients should carefully follow the instructions (“Flagyl ER,” 2021). For instance, for Bacterial Vaginosis (BV), which is one of the common conditions for Flagyl ER treatment, the general dosage is one tablet every day during the course of one week (“Flagyl ER,” 2021). Patients should intake the drug at least one/two hours before or after meals to ensure maximum efficiency. This dosage is appropriate for adults, and the drug should not be taken by children or pregnant women (“Flagyl ER,” 2021). Ultimately, Flagyl ER has multiple risks and side effects, meaning that patients should only use it when necessary and carefully follow dosage regulations.
Dosing Considerations
Among the precautions of Flagyl ER, it is crucial to note the threat of overdosage and hepatic/renal impairment limitations. Overdosage is a relevant risk of Flagyl ER intake, leading to multiple adverse effects, such as nausea, ataxia, and vomiting (“Flagyl ER,” 2021). Moreover, metronidazole overdose has been observed in several suicide attempts over the course of the drug history. Patients with hepatic impairments can intake Flagyl ER but should follow additional guidelines considering the dosage and immediately report to their physician in case of side effects (“Flagyl ER,” 2021).
If the hepatic impairment is severe, it is highly advisable to not use Flagyl ER or other medications with metronidazole. Similarly, patients with renal impairment can intake Flagyl ER, but their condition should be regularly monitored because metronidazole can exceedingly accumulate and cause adverse effects (“Flagyl ER,” 2021). In summary, patients should consult with their physicians and carefully estimate whether using Flagyl ER is necessary for treatment.
Conclusion
Flagyl ER is one of the medications that use metronidazole as an active component for the treatment of bacterial, parasitic, and protozoal infections. The only supply method is oral tablets, and patients should thoroughly consider the dosage because the drug is associated with multiple risks and the threat of overdose. Some demographic groups, including children and pregnant women, should not intake the medication. Ultimately, Flagyl ER is a highly effective nitroimidazole antibiotic, but its associated side effects and risks pose significant threats, and it is crucial to follow the dosage regulations.
References
Flagyl ER. (2021). RxList. Web.
Memon, N. (2021). What are names of nitroimidazoles drugs? RxList. Web.
Metronidazole – Drug summary. (n.d.). Prescribers’ Digital Reference. Web.