The following is a review of the article on the hydrogen-bonding complexes of 5-Azauracil and uracil derivatives. The article discusses the strong complexes formed when the derivatives of Uracil bond with complementary compounds such as 2, 4-dioxotriazine and adenine. They explore why the strong bonds are formed between these compounds while weak bonds are formed when 5-azauracil combines in an aqueous medium (Diez-Martinez, Kim, & Krishnamurthy, 2015). The researchers explain that the moieties formed when the different compounds combine have special bonds that interfere with those of water in aqueous form leading to the differences in strength.
The researchers discuss the process of solvation that is thought to affect the strength of the hydrogen bond formed between the different moieties (Diez-Martinez, Kim, & Krishnamurthy, 2015). This process is the interference between the bonds of water and the new hydrogen bonds formed when 5-azauracil combines in an aqueous medium. In an organic medium, solvation is absent. Consequently, there is no interference, and the hydrogen bonds are stable leading to the greater strength of these compounds in the organic medium. The researchers base their work on the role that solvation could have played in the origin of life. They state that the discriminating role of this solvent could have informed the selection of molecules utilized in oligomer formation during the process of life development.
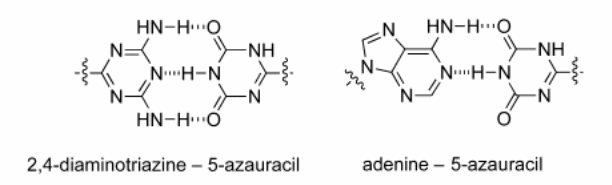
The researchers begin with an introduction of the different moieties and oligomers that are present in the DNA and the bonds present. In addition, they explain the differences in strength between some of the bonds formed between the different moieties. Solvation is an integral part of the natural selection process as water is thought to interfere with the selection of the different moieties and compounds that form the DNA. In fact, the researchers observed minimal interference between the ADA association with DAD bond partners when placed in chloroform (Diez-Martinez, Kim, & Krishnamurthy, 2015). However, the interference was greatly increased when the hydrogen bonds were put in aqueous medium.
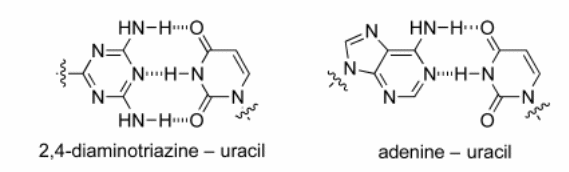
In the pairing studies, these researchers used spectroscopy to investigate the strengths of association. The other method used was self-association where the researchers investigated the NH proton shift at varying concentrations at the temperature of 298K. The proton shift in the hydrogen bond improves the strength of this bond. The third method used was cross-association where the researchers experimented with adenine and 2, 4-Diaminotriazine (Diez-Martinez, Kim, & Krishnamurthy, 2015). In organic solvent, the pairing of uracil and adenine is mediated through the Hoogsteen mode that is different from the Watson-Crick mode. The Watson Crick mode is shown in the combination of 2,4-diaminntriazines and 5-azauracil.
In the three methods describes, the researchers reported a weaker association of azauracil in water (Diez-Martinez, Kim, & Krishnamurthy, 2015). The explanation given for the weaker association is the pKa value of this compound that is close to the neutral pH. A nitrogen and argon atmosphere was used in the experiments. In addition, the researchers used Thin Layer Chromatography (TLC) alongside UV lamps and PMA. An ion trap mass spectrometer measured the different characteristics of the compounds and their mass spectra.
The researchers also conducted a study to determine the binding constant. In this procedure, they performed a binding study of each of the host molecules and monitored the effects using the NMR spectroscopy (Diez-Martinez, Kim, & Krishnamurthy, 2015). These researchers also performed a job plot procedure in CDCl3 where the molarity of the fluid was increased with spectrometer readings being taken. They also recorded the NH shift of the molecules and used this data to construct a job plot.
When synthesizing the derivatives, the researchers describe a complex process involving different pathways and reactants. They began with the creation of a suspension of the 6-chloromethyl-uracil and diisopropylthylamine. Into this mixture, they added bis-protected-pyrrolidine and stirred the resulting mixture at 60 0C. The mixture was stirred over a period of 24hours to allow for a perfect mix and elimination of the extra solvent. This process was followed by the further evaporation of the remaining solvent at a reduced pressure (Diez-Martinez, Kim, & Krishnamurthy, 2015). The resulting residue was re-dissolved in MeOH after which the researchers filtered the residue through a celite and silica bed. The same mixture of the solvent was used to wash the bed several times. Lastly, the researchers combined the filtrate and the washings and dried them to a solid mass that need no further purification.
The conclusion included that the bonding behavior of azauracil moiety in chloroform with its complementary counterpart has the same strength as uracil and adenine in chloroform (Diez-Martinez, Kim, & Krishnamurthy, 2015). The experiment showed that the base pairing propensity in water was mediated by the hydrogen bond in these compounds. Consequently, the properties of these compounds are determined by the moieties and the strength of the hydrogen bonds in aqueous media.
References
Diez-Martinez, A., Kim, E., & Krishnamurthy, R. (2015). Hydrogen-Bonding Complexes of 5‑Azauracil and Uracil Derivatives in Organic Medium. The Journal of Organic Chemistry, 80(1), 7066−7075