Introduction
The process of research and development (R&D) involves the design or improvement of a product, technology, or service. R&D is vital for business because it encourages innovation, helping a company generate revenue in the future and gain a competitive advantage (Hammar and Belarbi, 2021). In the pharmaceutical industry, R&D spending is required to obtain drug approvals and introduce products to the market (Grant et al., 2018). Congressional Budget Office (CBO) (2021) reports that in 2019, the industry’s spending on R&D reached $83 billion, which is ten times greater than the 1980s spending. A company’s investment in R&D depends on the expected revenue from a product, its ability to withstand disruption, and related policies regulating the production and supply of a drug.
The COVID-19 outbreak considerably affected the world economy and international trade, as it disrupted business operations and accelerated change. The pandemic caused major supply disruptions and challenged businesses to develop supply chain resilience (van Hoek, 2020). However, global pharmaceutical corporations experienced positive stock market growth during the pandemic (Kant, 2021). There is an increase in innovation in the infectious disease management field during the global fight against COVID-19 (GlobalData Healthcare, 2020). Due to the lack of effective treatment, the pharmaceutical industry has to match supply with demand by producing additional vaccine doses (Zimmer, 2021). Pfizer is one of the corporations benefitting from the COVID-induced clinical demand fueled by mass vaccination campaigns in high-income countries (Reed, 2021). A large share of the company’s R&D investments was directed towards the development of the coronavirus vaccine.
The scope of the following assessment includes the identification of the multinational company (Pfizer) and evaluation of its recent investment decision. The main objectives are to conduct thorough research to understand a company’s background and discover evidence to determine whether the investment decision can be justified. The primary method of investigation used in the paper is the literature research and online sources related to the topic. The technique involves the analysis of publicly available information from press publications and quantitative data from reports and financial statements. Additionally, a comparative analysis is employed to critically evaluate the company’s investment against R&D projects by other industry players. Finally, the methods of investment appraisal, such as net present value, profitability index, and accounting rate of return, will be discussed in the paper.
Company and Investment Identification
Pfizer (PFE) is a multinational research-based biopharmaceutical company with headquarters in New York City, NY (USA). It was established in 1849 and currently has 78,500 full-time employees (Pfizer Inc. (PFE), 2021). The stock price is $46.511, and the market capitalization value is $262.6 billion (BBC Business, 2021). The corporation is involved in developing, discovering, manufacturing, selling, and distributing pharmaceutical products (Reuters, 2021). Its overall market share is approximately 14.64% (PFE sales vs. its competitors Q2 2021, 2021). Pfizer’s international diversification strategy allowed it to improve its performance and market presence (Teramae et al., 2020). Moreover, the focus on specific healthcare products helped it avoid “the negative joint effect” of product/international diversification on performance (Castellani et al., 2018, p. 302). The company offers innovative biopharmaceutical solutions in over 100 developed and emerging markets in Africa, Latin America, Asia, and Middle East (Pfizer, 2018). Its main competitors are Novartis, AbbVie, Merck, and Eli Lilly, and Co.
The recent investment decision reviewed in the assessment is Pfizer’s R&D program involving the development of the BNT162b2 two-dose coronavirus vaccine. The pharmaceutical giant collaborated with the German biotechnology company BioNTech to develop the product and share financial obligations (BioNTech, 2018). The investment decision was justified because the COVID-19 pandemic fueled the demand for effective vaccines. Furthermore, the U.S. Food and Drug Administration (FDA) issued Emergency Use Authorization (EUA) for 19 major manufacturers, including Pfizer (Speights, 2021). BNT162b2 was the first project to be fully approved by the FDA and authorized for emergency use due to its 91% effectiveness (Debusmann, 2021). Thus, as a leader in drug approvals and successful pharmaceutical products, Pfizer analyzed the unprecedented demand for COVID-19 vaccines before investing in the project’s R&D.
The company and the investment decision were selected for the assessment for several reasons. Firstly, the COVID-19 implications for the healthcare sector and biopharmaceutical companies involve accelerated innovation and investment opportunities (RBC, 2021). Thus, it is worth examining the investment decision of Pfizer to understand the management of investments during the crisis. Secondly, the BNT162b2 investment project addresses the demand for COVID-19 protection and might be interesting for research in International Business Management (Kates, Rouw, and Michaud, 2021). Considering the predicted demand for coronavirus vaccines and booster shots in the following years, it is reasonable to assume that Pfizer might expect significant revenue growth (Carchidi, 2021). Finally, the critical assessment can provide helpful suggestions for strategies enhancing the financial feasibility of future projects in the global market. Investment appraisal helps companies evaluate the rationality of an investment decision, introduce relevant products, and improve stock growth potential.
Critical Evaluation of the Investment Decision
The critical evaluation of an investment decision will be based on appraisal methods. Cavalcante and Rocha (2018) suggest that “investment decisions aim to identify real assets whose value is higher than the cost of acquisition” (p. 125). Pfizer collaborates with governments, healthcare providers, patient foundations, and academic experts to expand its R&D ecosystem (Pfizer, 2021a). The BNT162b2 investment is a part of Pfizer’s R&D efforts in response to the COVID-19 crisis (Segal, 2021). The methods of investment appraisal will be discussed below and applied to evaluate the investment decision of the selected company critically.
Net Present Value
Net present value (NPV) is a method used to measure a project’s cash flow merit demonstrating how the value of future cash inflows relates to the value of expected outflows. NPV is viewed as “the most reliable method for capital budgeting decision-making” (Cavalcante and Rocha, 2018, p. 125). According to Pike et al. (2018), investments with positive NPV values increase a firm’s wealth. NPV is an important method for decision-makers for several reasons. First, managers should act in the shareholders’/owners’ best interests by maximizing cash flow over time, which is reflected in the current rate of interest based on NPV. Second, the NPV approach suggests undertaking projects “to the point at which the marginal return on the investment is equal to the rate of interest on equivalent financial investments” (Pike et al., 2018, p. 88). Finally, NPV eliminates the need for managers to investigate shareholders’ risk preferences and specific consumption time patterns due to well-functioning capital markets.
The investment decision can be critically evaluated by calculating the project’s NPV. Cash flows are discounted by a company’s specified discount rate (CFI, 2021). Pfizer’s discount rate is in the range of 5%-6% and an average of 5.5%, while the initial cost of investment is $1 billion (5Y DCF growth exit, 2021). It is reasonable to include the data from Year 1 (2020) and Year 2 (2021) to determine the NPV of the project with a lifespan of 2 years. The formula is NPV=Xt/(1+k)t – I, where ‘X’ is cash flow, ‘k’ is discount rate, I is the investment’s initial cost, and n is a period number (e.g., year). The cash flow is $14,4 billion for Year 1 and $23,5 billion for Year 2 (Pfizer Inc. (PFE), 2021). Thus, the project’s NPV is (14,4 divided by 5.5) plus (23,5 divided by 5,52) minus 1. The investment’s NPV of $2,37 billion is below the NPVs of rival products launched by Roche ($99.78 billion), AbbVie ($76.44 billion), and AstraZeneca ($45.5 billion) (Kansteiner, 2021). The positive value suggests that the project’s expected earnings exceed its anticipated costs.
Profitability Index
The profitability index (P.I.) is a method used to evaluate investment projects. P.I. refers to the ratio of the present value of future cash flows to the current value of the initial investment that provides advice on the feasibility of an investment decision (Hillier, 2020). Pike et al. (2018) state that P.I. greater than 1.0 is considered acceptable. For instance, the P.I. of the company’s vaccine program can be calculated by dividing its cash flow ($23,5 billion) by the costs of the project’s R&D ($1 billion) (Baker, 2021). Comirnaty project’s P.I. may be calculated by dividing its NPV ($2,37 billion) by the investment amount ($1 billion) and equals 2,37. The value is greater than 1.0, which suggests that the investment is profitable.
Accounting Rate of Return
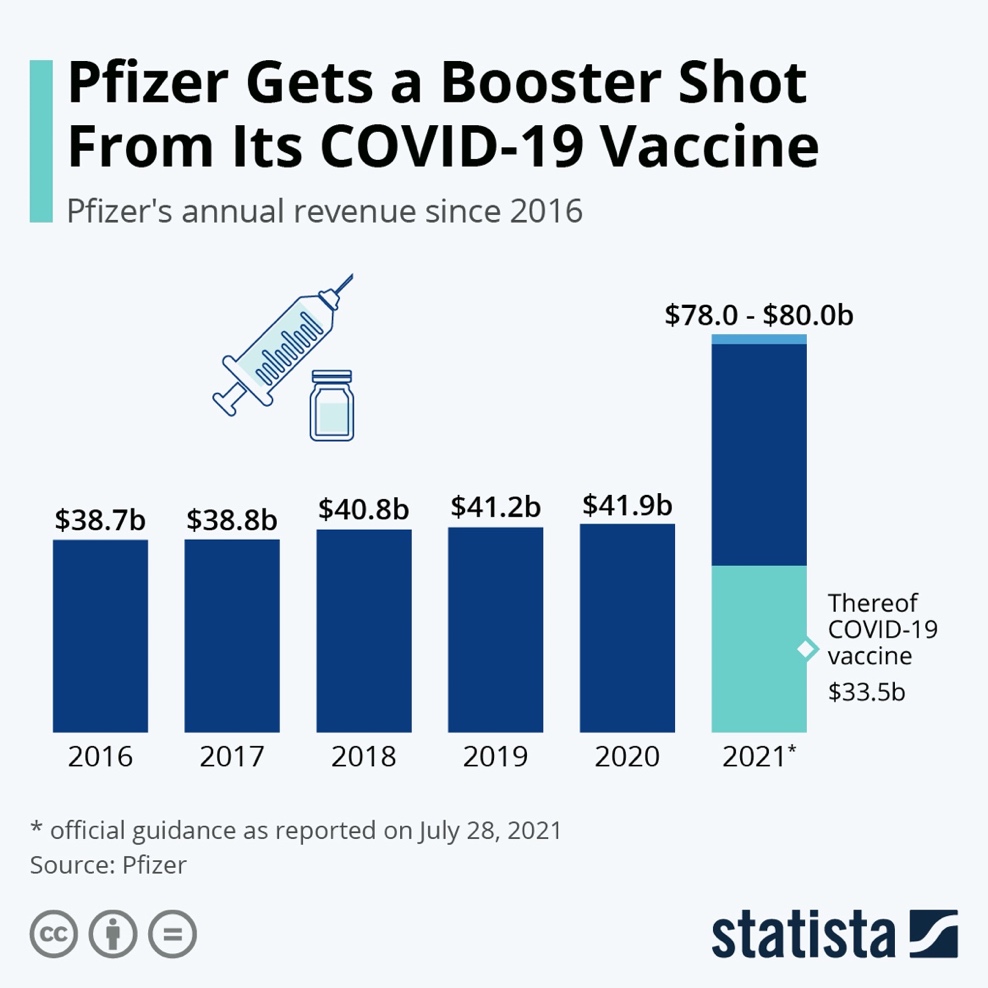
The accounting rate of return (ARR) is a percentage reflecting the measurement of project profitability over the asset life. ARR can be calculated by dividing the project’s average annual profit by the initial R&D investment and multiplying the amount by 100. The graph (Figure 1) below demonstrates that Pfizer’s yearly revenue has significantly increased since 2016 following the implementation of the BNT162b2 vaccine project in 2021. The diagram reveals that the average estimated annual profit from Pfizer’s coronavirus vaccine in 2021 is $33.5 billion. The gain can be calculated by deducting total costs (40% of the revenue, or $13.4 billion) from the annual income ($33.5 billion) and equals $20.1 billion (Pfizer, 2021b). Therefore, the ARR is 3,350% (33.5 divided by 1 and multiplied by 100). Ledley et al. (2020) note that the average profitability of large pharmaceutical companies is significantly higher in comparison with non-pharma firms. The calculations related to Pfizer’s project reflect the substantial rate of return that proves the assumption about the profitability of the pharmaceutical industry.
Factors Pertaining to the Investment Decision
The factor pertaining to the investment decision should be reviewed to enhance the understanding of the reasoning behind the investment decision. The main factor related to the R&D project is confidence associated with the potential profitability of the end product. Mahlich and Yurtoglu (2018) claim that “the median profitability of the pharmaceutical firms has been persistently above the median profitability of firms from other industries” (p. 16). It should be noted that the industry “belongs to both the most research-intensive and to the most advertising intensive” fields, with about $57.5 billion spent annually on advertising (Mahlich & Yurtoglu, 2018, p. 16). Moreover, Pfizer’s international diversification strategy aims to introduce innovative solutions worldwide, which is why the corporation included the supply of 100 million doses to its consumers in Africa (Oxfam, 2021). Thus, the decision to invest in the R&D project might be explained by the company’s expectations to increase revenue from vaccine sales and improve the company’s global presence as a part of its diversification efforts.
Another factor is the change in demand and market conditions creating opportunities for the development and approval of new products. The COVID-19 pandemic had a positive impact on the financial returns of pharmaceutical companies due to the emergency status accelerating the process of drug approval and marketing authorization by regulatory agencies (European Medicines Agency, 2021). The total value of recent approvals is $17.41 billion contributing to 11.54% of the net present value of all Pfizer’s products ($150.87 billion) (Kansteiner, 2021). Market conditions shaped by the outbreak encourage “fast decision making within pharma companies and a high tolerance for investment risk” (McKinsey, 2021). Additionally, Oxfam (2021) reports that major pharmaceutical companies have monopolized vaccine production, and the growing competition could impact investment decisions. Therefore, the R&D of the vaccine was financially justified because it was expected to rapidly enter the market to temporarily compensate for the lack of effective drugs for the treatment of the disease.
Investment Financing
The analysis of the project’s financing is vital for understanding the feasibility of the investment. Due to the devastating impacts of the COVID-19 outbreak on world economies, governments invested about $10 billion to fund pharmaceutical R&D, as vaccines prevent further economic losses (Paton & Lauerman, 2021). Pfizer’s primary competitor, Moderna, received $4.1 billion from the American budget to produce the vaccine. Pfizer secured a $2 billion deal with the country to supply vaccine doses but rejected the development subsidies from the U.S. government (Paton & Lauerman, 2021). LaMattina (2021) suggests that Pfizer denied the funding opportunity because “extra bureaucracy would slow down its efforts.” Instead, the company received a foreign sponsorship for its BNT162b2 project. Collaboration agreement with a German mRNA-specializing company BioNTech allowed Pfizer to divide development costs and receive substantial funding from the German government through the partnership with the local vaccine manufacturer. The diagram (Figure 2) below shows the distribution of the investment from various financing sources, including government, non-profit, and private donors, in Pfizer and its competitors.
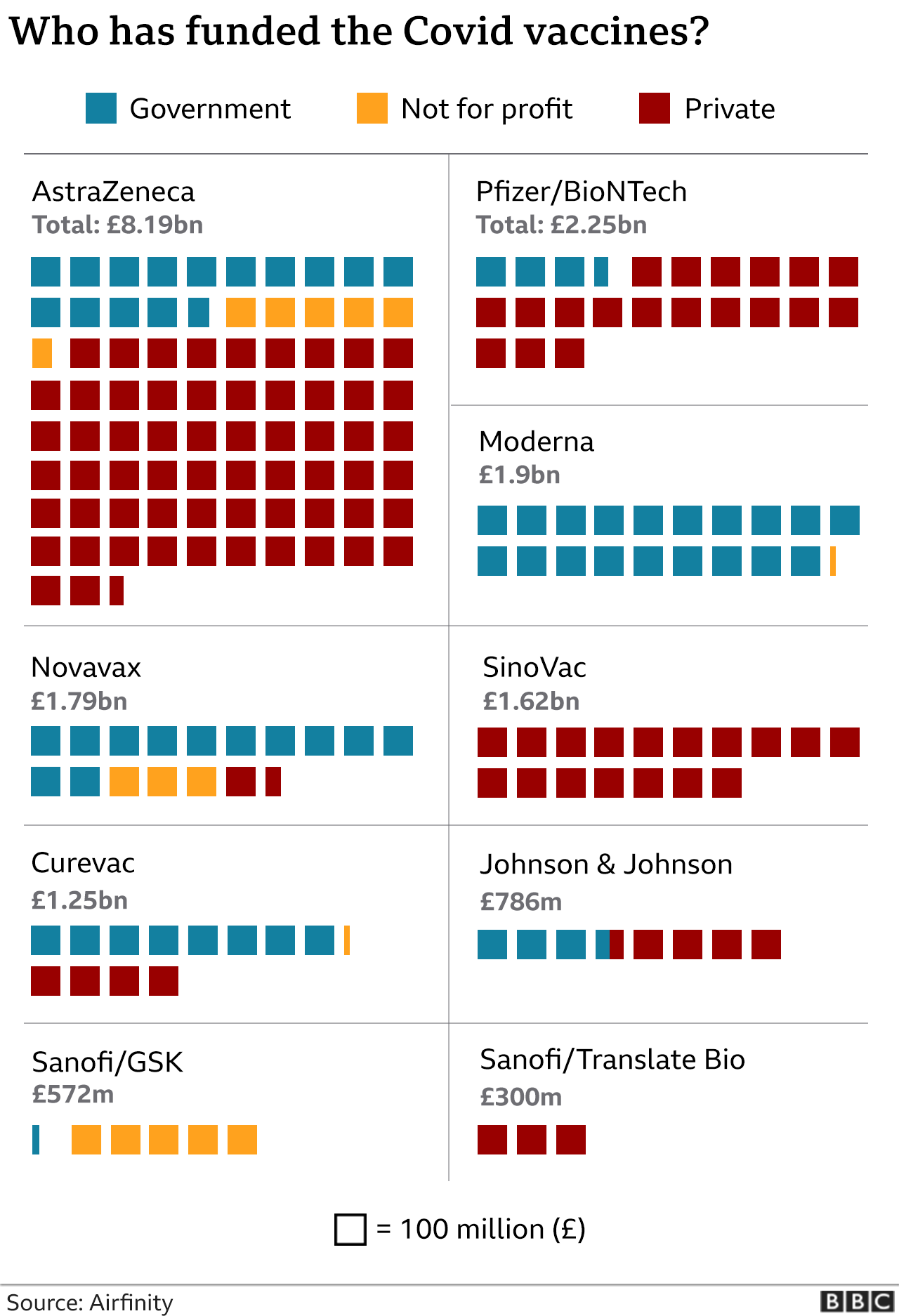
The diagram suggests that investment financing covered the amount required for Pfizer’s project. The vaccine’s R&D expenditures of about $1 billion are considerably below the vaccine’s expected annual revenue (Baker, 2021). The vaccine is estimated to reach $33.5 billion in sales in 2021 versus $19 billion expected from Moderna, while AstraZeneca refused to gain profit from their product during the COVID-19 crisis (Paton & Lauerman, 2021). Thus, the adequate funding for Corminaty’s R&D and the expected revenue from the project, which is substantially higher than the competitors’ estimates, can justify Pfizer’s investment decision.
Recommendations
The recommendation regarding the investment project will be based on the appraisal results and relevant scholarly studies. The investment’s NPV value ($2,37 billion) is below the net present values of rival products launched by Roche ($99.78 billion), AbbVie ($76.44 billion), and AstraZeneca ($45.5 billion) (Kansteiner, 2021). The main recommendation for the R&D decision discussed in the paper is to avoid over-confidence bias. The type of bias can be observed in the company’s focus on a single product (the coronavirus vaccine) and the disproportionate allocation of resources on R&D associated with this product. Ahmad et al. (2021) maintain that over-confidence bias has a “significant impact on investors’ decision-making” (p. 1077). Thus, it would be reasonable to decrease the investment in the Comirnaty vaccine and support the diverse portfolio of biopharmaceutical products.
Another recommendation is related to the inflated cost of vaccination, which might be caused by Pfizer’s refusal to accept government subsidies and monopolization of the mRNA vaccine market. The price of a dose (from $6.75 to the African Union to $28 for Israel) makes it unavailable for developing countries (Oxfam, 2021). It also raises ethical concerns impacting the public image of the biopharmaceutical corporation. Therefore, accepting government support for the R&D investment would have decreased the cost of the vaccine and improved its global availability.
Conclusion
To sum up, investments in R&D are common in the pharmaceutical industry, as they help companies promote innovation, potentially increase revenue, and gain a competitive advantage. The COVID-19 pandemic considerably affected the global economy accelerated the change process, and encouraged innovation. The paper discusses the investment decision related to Pfizer’s R&D project produced in collaboration with a German BioNTech company, which attracted financing from the German government. The investment decision was selected because of COVID-implications for international business and the pharmaceutical industry, demand for vaccine products, and the value of investment appraisal for enhancing projects’ feasibility in the global market.
Pfizer’s core financial data used in the paper and key considerations involved in the investment decision are summarized in the table below (Figure 3).
Figure 3: Pfizer’s financial data
The investment appraisal revealed that the project’s NPV is a positive value of $2.37 billion that is below the NPVs of the vaccine products launched by competitor companies (Roche, AbbVie, and AstraZeneca). The calculation of the investment’s P.I. resulted in a value of 2,37 that is considered appropriate and indicates the project’s profitability, as it is greater than 1.0. Furthermore, the investment’s ARR was assessed to estimate the profitability of the BNT162b2 program over its lifespan. The result of 3,350% is based on the project’s annual profit and the amount of R&D investment. It is consistent with the reported profitability of major pharmaceutical companies and the industry in general. In addition to the investment appraisal methods applied to the project, factors pertaining to the decision were considered. The factors included the company’s confidence in the project’s profitability, its international diversification strategy related to vaccine and drug solutions, the growing demand for effective coronavirus vaccines. Moreover, fast decision-making became available due to timely approvals by the FDA and EUA.
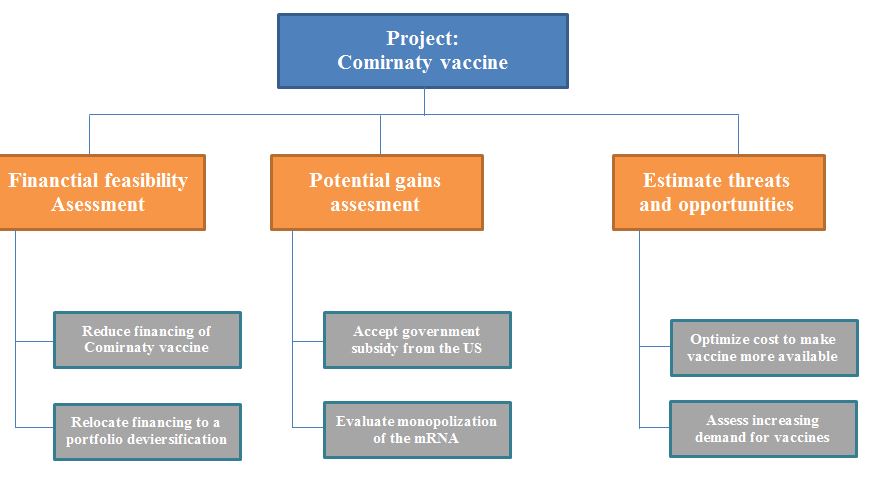
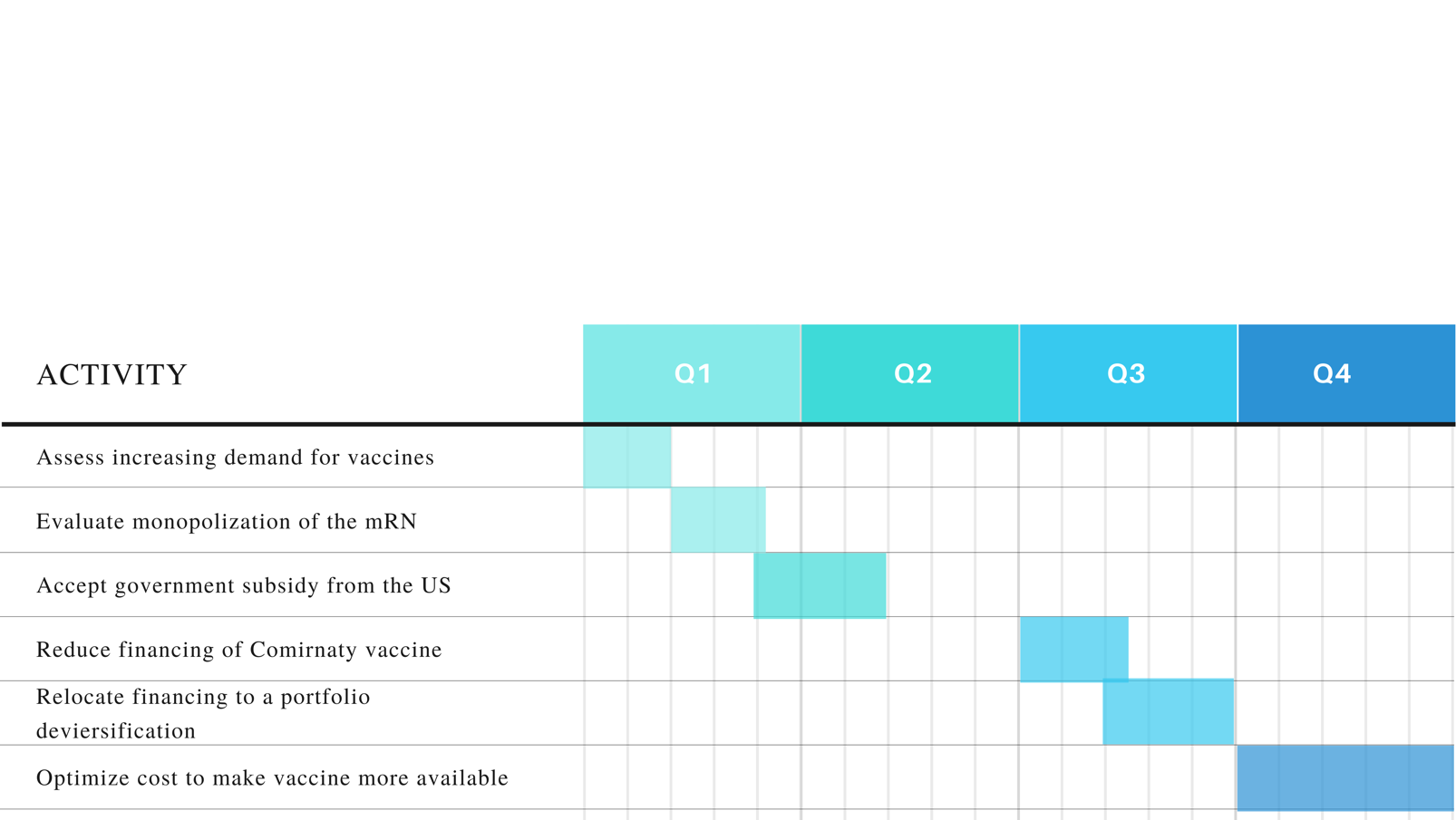
The recommendations for the projects consider the investment appraisal data and suggestions from relevant scholarly studies. Pfizer should void over-confidence bias and decrease the financing for the Comirnaty vaccine since the project’s NPV is below the competitor product’s NPVs. It is also recommended to optimize the cost of the vaccine dose, which makes it unavailable for consumers in developing countries and raises ethical concerns. Government subsidies should be considered as a solution for decreasing the product’s price without negative revenue implications. The activities are broken down in the WBS chart (Appendix 1) and the proposed course of action is established in the Gantt chart (Appendix 2) Therefore, the financial evidence suggests that the decision to invest in the coronavirus vaccine was justified but may be improved to address economic and ethical issues.
Reference List
Ahmad, R. et al. (2021) ‘The relationship among overconfidence, economic expectation, social factors and investment decision making behavior with the mediating and moderating effects’, Journal of Contemporary Issues in Business and Government, 27(2), pp. 1075–1088.
Baker, B. (2021) Pfizer and Biotech profiteering exposed – More than enough is enough. Web.
BBC Business (2021) Pfizer Inc. Web.
BioNTech (2018) BioNTech signs collaboration agreement with Pfizer to develop mRNA-based vaccines for prevention of influenza. Web.
Carchidi, A. (2021) At less than $50, could Pfizer reach $100 before 2025? Web.
Castellani, D., Narula, R., Nguyen, Q.T.K., Surdu, I. & Walker, J.T. (2018) Contemporary issues in international business. Cham: Palgrave MacMillan.
Cavalcante, L. R. and Rocha, C. H. (2018) ‘Investment appraisal and the choice between continuous and discrete cash flow discounting’, Exacta, 16(4), pp. 125–134.
CFI (2021) What is the NPV formula? Web.
Congressional Budget Office (2021) Research and development in the pharmaceutical industry. Web.
Debusmann, B. (2021) Pfizer becomes first Covid vaccine to gain full FDA approval. Web.
5Y DCF growth exit (2021) Web.
GlobalData Healthcare (2021) The impact of big pharma on Covid-19. Web.
Grant, K. et al. (2018) ‘Research and development spending and technical efficiency: Evidence from biotechnology and pharmaceutical sector’, International Journal of Production Research, 58(20), pp. 6170–6184.
Hammar, N. and Belarbi, Y. (2021) ‘R&D, innovation and productivity relationships: Evidence from threshold panel model’, International Journal of Innovation Studies, 5(3), pp. 113–126.
Hillier, D. (2020) Corporate finance, 4th edn. New York, NY: McGraw-Hill Education.
Hooker, L. and Palumbo, D. (2020) Covid vaccines: Will drug companies make bumper profits? Web.
Kansteiner, F. (2021) 8. Pfizer. Web.
Kant, K. (2021) The second wave: Pharma stocks make a comeback as Covid-19 cases rise. Web.
Kates, J., Rouw, A., and Michaud, J. (2021) Supply vs demand: Which states are reaching their COVID-19 vaccine tipping points? Web.
LaMattina, J. (2021) Taxpayer funded research and the Covid-19 vaccine. Web.
Ledley, F. D. (2020) ‘Profitability of large pharmaceutical companies compared with other large public companies’, JAMA, 329(9), pp. 834–843.
Mahlich, J. and Yurtoglu, B. B. (2018) ‘Returns on different types of investment in the global pharmaceutical industry’, Managerial and Decision Economics, 40, pp. 16–36.
McKinsey (2021) COVID-19 implications for business. Web.
Oxfam (2021) Vaccine monopolies make cost of vaccinating the world against COVID at least 5 times more expensive than it could be. Web.
Paton, J., & Lauerman, J. (2021) When lifesaving vaccines become profit machines for drugmakers. Web.
PFE sales vs. its competitors Q2 2021. Web.
Pfizer (2018) Emerging markets. Web.
Pfizer (2021a) R&D ecosystem. Web.
Pfizer (2021b) Pfizer reports second-quarter 2021 results. Web.
Pfizer Inc. (PFE) (2021) Web.
Pike et al. (2018) Corporate finance and investment: Decisions and strategies. 9th edn. London: Pearson.
RBC (2021) The impact of COVID-19: A catalyst for change, innovation and investment. Web.
Reed, T. (2021) In the COVID-19 vaccine market, the problem has always been demand, not supply. Web.
Reuters (2021) Pfizer Inc. Web.
Richter, F. (2021) Pfizer’s annual revenue [Image]. Web.
van Hoek, R. (2020) ‘Research opportunities for a more resilient post-Covid-19 supply chain – closing the gap between research findings and industry practice’, International Journal of Operations & Production Management, 40(4), pp. 341-355.
Segal E. (2021) Conflicting messages about Pfizer booster shot creates confusion in Covid crisis. Web.
Speights, K. (2021) Investing in coronavirus vaccine stocks. Web.
Teramae et al. (2020) ‘International strategy for sustainable growth in multinational pharmaceutical companies’, Sustainability, 12, pp. 1–14.
Zimmer, C. (2021) How the search for Covid-19 treatments faltered while vaccines sped ahead. Web.
Appendix 1: WBS Chart
Appendix 2: Gantt chart
Footnotes
- 1 – As of September 8, 2021.