Cytomegalovirus, also known as CMV, is the leading cause of congenital infection, with morbidity and mortality at birth and sequelae (Lazzarotto, et al., 2011). Each year, approximately 1–7% (Rev Med Virol 2010; 20: 311) of pregnant women acquire a primary CMV infection. Of these, about 30–40% transmit the infection to their fetuses. Thousands of children are born with or develop permanent disabilities such as hearing loss, vision loss, or motor and cognitive deficits from congenital cytomegalovirus infection (Fowler, 2018). Some newborns have symptoms at birth which consist of seizures, an enlarged liver and spleen, retinitis, jaundice, and hearing loss. Congenital CMV infection is the leading non-genetic cause of sensorineural hearing loss (SNHL) in early childhood, accounting for 21% of children with hearing loss at birth and 24% of those with hearing loss at 4 years of age (Ahmed, 2017). Congenital CMV is the leading non-genetic cause of children experiencing deafness (Lazzarotto et al., 2011). CMV is part of the family of herpes viruses and is very common.
As a Clinical Research Coordinator, I have observed newborns experience hearing loss after birth. A large number of parents have never heard of CMV and do not believe that their child could have possibly been exposed to this virus. Human cytomegalovirus is a leading cause of congenital infections worldwide. In the developed world, following the virtual elimination of circulating CMV, it is the commonest nongenetic cause of childhood hearing loss and an important cause of neurodevelopmental delay (Gupta et al., 2018). A mother can contract this virus and not know due to being a healthy adult, this virus is similar to a common cold.
The purpose of this research is to provide a better understanding of analysis within the asymptomatic population. The goal is to increase the amount of knowledge regarding Cytomegalovirus and how it relates to the Valganciclovir treatment as displayed on the CDC website. North Carolina is moving towards the standard of care CMV testing but has not rolled
out a process due to the state budget. The state should understand the importance of this virus and include it in standardized testing within the next few years. This test can make a huge difference in the lives of others and that is the main focus of the healthcare system, ensuring that all babies can live a quality life within the United States. The worldwide neglect of this problem is underscored by the continued lack of awareness of congenital CMV among health care workers and the public (Manicklal, et al).
Babies get this virus through the placenta from their mother. This virus can also spread through infected breastmilk, which means about 1 in 200 babies are born with CMV. Only around 1 in 5 with this virus will get sick or have long-term health issues (ValEar). When a child has contracted CMV, there are oftentimes no symptoms until later on in life. CMV testing is not a standard of care procedure in North Carolina. Some mothers take their children home and find out years later that their child has developed hearing loss due to CMV. There are not enough physicians that have proper knowledge of this virus because the metrics are so low in asymptomatic babies. The urgency for treatment is not presented unless the child has symptoms of CMV.
Testing
Traditionally, the culture of the urine or saliva has been considered the most reliable method for the diagnosis of congenital CMV infection. Urine is typically collected from infants for viral culture using sterile urine bags, however, there are inherent difficulties associated with this collection method (Ahmed, 2016). The CHIMES study was conducted at my site with the PI that I currently work with. A variety of methods have been evaluated for use in the diagnosis of congenital CMV infection on the basis of saliva, urine, and dried blood spot specimens obtained from newborns (Yamamoto et al., 2006). Culture-based testing of urine and saliva specimens has been the standard method to identify infants with congenital CMV infection. (Yamamoto, et al., 2006).
The CMV collection method that we are currently using for newborns consists of a mouth swab for 25 seconds. We have to follow protocol and wait at least 30 minutes after the child has breastfed. The breastmilk could cause a false positive due to mom having the active virus inside of her breastmilk. With all of its bioactive, immunological, anti-inflammatory and nutritive components – it is generally believed to be the most beneficial form of nourishment for human infants. However, breastmilk is also a mode of cytomegalovirus transmission to infants (Reynolds, 1980). The risk for long-term outcomes appears to be highest in infants born to mothers with primary infection in the first half of pregnancy (Gupta, et al., 2018). The lab confirms if the child has been exposed to CMV by testing their saliva and urine. Reliable methods to screen newborns for congenital cytomegalovirus (CMV) infection are needed for identification of infants at increased risk of hearing loss (Boppana, et al., 2010). The standard assay for newborn CMV screening is rapid culture performed on saliva specimens which are obtained at birth. Two alternatives the first one is real-time polymerase-chain-reaction (PCR) based testing of a liquid-saliva, the alternative is dried-saliva specimen obtained at birth, these have both been developed (Bopanna, et al., 2011).
The CHIMES study conducted testing and 177 of the 34,989 infants tested positive for CMV (Boppana, et al., 2011). Liquid saliva specimens were processed for rapid culture and PCR assay for this study. The dried saliva we processed, and DNA extractions were taken from each tube. The majority of infants with congenital CMV infection will not be identified by means of clinical examination during the newborn period (Boppana, et al., 2011). “Although traditional virus isolation from saliva or urine specimens in tissue culture is considered the standard method for identification of infants with congenital CMV infection, it is not amenable to mass screening (even when modified to produce rapid results) because it is labor- and resource-intensive and requires tissue culture facilities.” (Boppana,et al., 2010).
Prevention
Expecting mothers should take proper precautions and receive testing for CMV. This would allow the mother to know if their child could possibly be exposed to the virus. Infection with CMN can occur in pregnant woman by a non-primary infection, and the virus can be reactivated by reinfection from a different strand (Lazzarotto, et al., 2011). Primary infections that effect the mother have a great impact on the fetus. CMV is difficult to prevent because the virus is very common (Lazzarotto, et al., 2011). Screening for CMV is available for mothers expecting, along with a counseling that is provided for parents that are not sure of the outcomes of children with the virus (Revello & Gerna, 2002).
Currently, the most important strategy to reduce the risk of congenital CMV infection is the hygiene counseling of pregnant women (Pediatr, 2017). There is CMV testing that is available for mothers during pregnancy. Once an expecting mother shows seroconversion or signs of an active CMV infection, there are no established procedures to reduce the risk of transmission to the child (Pediatr, 2017). This is an issue that needs to be addressed within the next decade.
Many women and even physicians are not aware of the risk for recurrent infection during pregnancy, despite preexisting immunity. Therefore, avoiding the exposure of pregnant women to CMV through behavioral changes should be recommended for seronegative, as well as for seropositive, pregnant women (Pediatr, 2017). This explains why there are so many patients that have no idea about CMV. The education of this virus will help prevention. There are ways that mothers protect their children from contracting HIV, and I believe that CMV is on the same level of importance. A child could possibly lose their hearing and have developmental problems later on in life due to the health care innovative system not focusing on CMV.
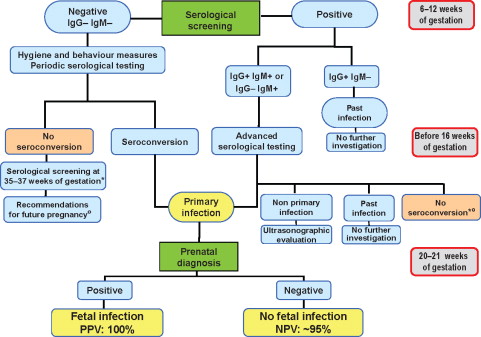
The figure below is an example of the susceptible Italian women that received information about CMV and preventative measures that they could take. The women were monitored for CMV antibodies at 18 weeks of gestation and also during delivery (Revello, 2015). The controlled study provides evidence that a primary prevention strategy based on the identification and provision of adequate information to susceptible pregnant women at risk for primary infection is highly effective in reducing the rate of maternal primary CMV infection (Revello, 2015).
Providing pregnant mothers with information about CMV and the importance of hygiene during pregnancy is vital. The figure below is proof that when addressing the issue ahead of time and informing the public can change the outcome. When walking into a room and explaining CMV to a mother for the first time after delivering her child is heartbreaking. Information that should’ve been provided by their OBGYN has now been delayed and provided to a mother by a research coordinator. We are working diligently to educate nurses on CMV so that when we walk into the patient’s room, they are not hearing about this virus for the first time.
Diagnostics Challenges
Due to the prevalence of subclinical forms of the course of congenital cytomegalovirus infection, its diagnosis is often delayed, and this, in turn, leads to the development of many complications. With a primary infection in a pregnant woman, viral DNA can be detected in fetoplacental tissue using PCR. If fetal pathology is detected during an ultrasound scan at the end of the second trimester, it is advisable to carry out cordocentesis or placenta biopsy, which, in addition to isolating the virus DNA by PCR, makes it possible to quickly determine the karyotype. Only 60–70% of cases are able to prove the presence of IgM antibodies in the fetal blood (Dietrich & Schieffelin, 2019). Since with negative PCR, neither the development of CMVI in the fetus at a later point in time, nor false-negative results can be reliably excluded, and further serological and sonographic monitoring is required in these cases (Dietrich & Schieffelin, 2019).
Diagnosis of primary CMVI is usually based on the determination of seroconversion, the presence of a high titer of specific IgM, or a four-fold increase in IgG titer. Due to the fact that the moment of seroconversion is difficult to diagnose, IgM are the main markers for verification of acute infection. However, in some patients, IgM persist for a very long time, which leads to overdiagnosis of the primary infection. In this case, the determination of the avidity of IgG class antibodies comes to the forefront, since low-avidity antibodies are detected during a recent infection, and high-avidity antibodies are detected during a long-term infection. Thus, the use of the avidity test allows confirming or excluding the presence of primary CMVI.
The “gold standard” is the definition of anti-CMV IgG with low avidity in maternal blood and the detection and quantification of the virus in amniotic fluid using real-time polymerase chain reaction (PCR) (Toriyabe et al., 2017). Recently, however, there has been much discussion of recommendations for the determination of anti-CMV IgM in the blood of mothers and newborns, the study of PCR in maternal blood, urine and amniotic fluid, as well as the invasive amniocentesis procedure itself, which can lead to rupture of membranes, intrauterine infection or miscarriage.
It is shown, for example, that true primary CMVI is diagnosed only in 20–25% of pregnant women with positive results for anti-CMV IgM. This is due to the fact that anti-CMV IgM can persist for 6–9 months after the initial infection, and can also be detected with latent reactivation. In practice, it is rarely possible to trace true seroconversion. On the other hand, the results of PCR and antigenic tests in maternal blood and urine do not correlate with the risk of congenital infection or the severity of fetal disease (Gantt et al., 2017). In 2016, Japanese scientists proposed a non-invasive approach to predicting congenital CMVI of the fetus. After analyzing the data of laboratory and instrumental examination of 300 anti-CMV IgM positive pregnant women, they found that the most effective is the detection of ultrasound signs of fetal developmental abnormalities in combination with the positive results of PCR secretion of the cervix of the pregnant woman. In this case, according to the researchers, doctors have the opportunity to start early antiviral treatment of the newborn and thus improve the neurological prognosis of congenital CMVI (Gantt et al., 2017).
Revello and Gerna claim to consider the disgnosis as the most controversial issues, in particular, how the problem of HCMV infections in pregnancy is perceived by the scientific community (Revello & Gerna, 2002). In their right opinion, “prenatal testing represents a very important option in the case of primary infection during pregnancy, as documented by the acceptance by the majority of women to whom it was presented” (Revello & Gerna, 2002, p. 705).
In addition to blood, cytomegalovirus can be detected in other body fluids – saliva, urine, breast milk, etc., which has been of particular interest to researchers in recent years. For example, the possibility of using various biological media for PCR screening of newborns to diagnose congenital forms of cytomegalovirus infection is widely studied (Boppana et al., 2011). Detection of cytomegalovirus DNA in samples of saliva and urine in children in the first days of life, even without taking into account its amount, may indicate a cytomegalovirus disease. However, in children older than 1 month of life, only quality detection of virus DNA in saliva and urine to determine the cytomegalovirus status is completely insufficient, since it is known that the frequency of virus excretion in saliva and urine in clinically healthy individuals is from 10 to 50% depending on age (Marsico & Kimberlin, 2017). This issue continues to be poorly studied, although some general trends in virus excretion in saliva and urine can be distinguished, both in patients and in children without clinical manifestations of cytomegalovirus infection.
Treatment of CMVI in Newborns
Despite the toxicity of antiviral anti-CMV drugs when a child develops a life-threatening manifest generalized CMVI, the use of ganciclovir and valganciclovir is indicated and necessary after a preliminary preliminary risk and benefit analysis (Kimberlin et al., 2015). Ganciclovir was first used in the treatment of newborns in the late 1980s; subsequently it was repeatedly shown that in general it is safe, well tolerated by children and effective especially for CMV damage to the central nervous system and eyes. The drug is prescribed in a dose of 5-6 mg/kg twice a day for an average of 6 weeks.
Limitations of the use of ganciclovir in practice are associated not so much with toxicity as with the need for permanent catheters for its infusion. An alternative to the invasive use of ganciclovir was the introduction of valganciclovir, a randomized study of the effectiveness of which was completed in 2015 (Rawlinson et al., 2017). According to Consensus 2017, treatment with valganciclovir (monovinyl ether is a prodrug of ganciclovir with high oral bioavailability) is indicated for children with moderate congenital CMVI – 16 mg/kg two times a day, up to (but no more) 6 months (Rawlinson et al., 2017).
During treatment, neutrophil control is mandatory (weekly up to 6 weeks, then at week 8, then monthly) and transaminases (monthly). An audiological examination must be carried out once every 6 months – up to 3 years, then – in adolescence (10-12 years). Antiviral therapy is not recommended for newborns with asymptomatic and mild congenital CMVI, as well as with isolated sensorineural deafness (Goderis et al., 2014).
Managing CMV
There are long-term outcomes of this virus and the treatment is given to the child for 6 weeks. It is also important to monitor the newborn during the time of antiviral therapy. Antiviral therapy of symptomatic congenital CMV infections, regardless of severity, clearly improves hearing ability and neurodevelopmental outcome when commenced in the first weeks of life (Pediatr, 2017).
The neglect of congenital CMV infection in the developing world reflects not only delayed onset of sequelae but also competing for health priorities in such populations. Given that early detection of hearing loss can limit long-term disabilities, PCR-based newborn screening to identify those at risk of sequelae deserves consideration (Gupta, et al., 2018). There are important factors that are continuing to be overlooked within the infectious disease population.
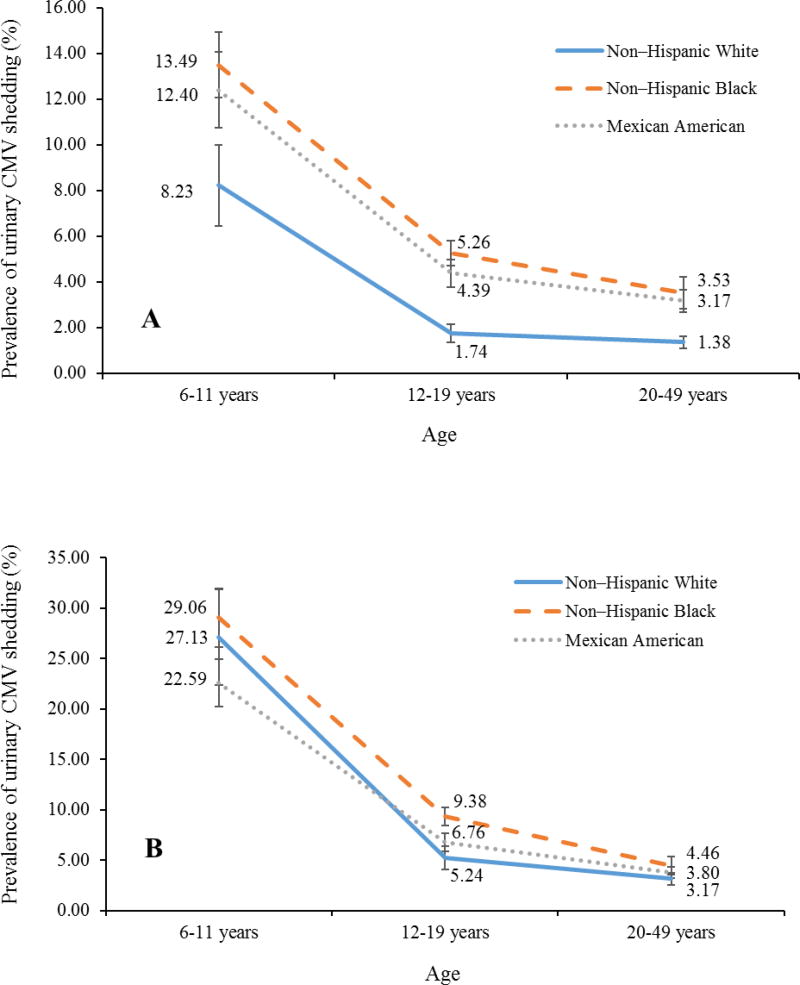
Urinary shedding plays a major role in the transmission of CMV the figure below is an example of the statistics that were found during a study. This study consists of 6,828 specimens tested from CMV positive subjects. There is a trend in the decreasing of shedding with age. There are three ethnic groups and the lowest was observed in non-Hispanic white individuals with higher household income and education levels. CMV positive subjects are displayed in the B chart. This figure allowed me to gain a better understanding of the importance of education and how much of a change the entire healthcare system would have by providing information about Cytomegalovirus.
Conclusions
Real-time PCR assays of both liquid- and dried-saliva specimens showed high sensitivity and specificity for detecting CMV infection and should be considered potential screening tools for CMV in newborns
SAS will be used to help gather a better understanding of male to female ratio using the t-test. Variables used will be male and female. However, it should be noted that there are numerous publications that provide other “cut-off” values. It is obvious that the “cut-off” values of the viral load can be different for various biological environments, have age- and sex-related features, and also depend on the set of primers in a particular laboratory. Thus, taking into account all the above, it becomes clear that for the application of the results of PCR diagnostics of cytomegalovirus infection in children, the clinical interpretation of the values of the viral load is especially important.
References
Advocacy & Policy (n.d.). 2020, Web.
Babies Born with Congenital Cytomegalovirus (CMV) (2020). Centers for Disease Control and Prevention. Web.
Berger, S. (2020). Cytomegalovirus infection: Global status. Nashville, TN: Gideon.
Boppana, M.D., Shannon, A., Ross, M., Shimamura, M., April, L. Palmer, M.D.,… , Britt, W. (2011). Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. The New England Journal of Medicine, 364, 2111-2118.
Boppana, M.D., Shannon, A., Ross, M., Novak, Z. (2010). Dried Blood Spot Real-time Polymerase Chain Reaction Assays to Screen Newborns for Congenital Cytomegalovirus Infection. JAMA, 303(14), 1375-1382.
Diar, H. A., & Velaphi, S. (2014). Vol. 8:133-137 Characteristics and mortality rate of neonates with congenital cytomegalovirus infection. Web.
Dietrich, M. L., & Schieffelin, J. S. (2019). Congenital cytomegalovirus infection. Ochsner Journal, 19(2): 123-130.
Gantt, S., Bitnun, A., Renaud, C., Kakkar, F., Vaudry, W. (2017). Diagnosis and management of infants with congenital cytomegalovirus infection. Pediatrics & Child Health, 22(2), 72-74.
Just another WordPress site (n.d.). 2020, Web.
Loss in Children With Asymptomatic Congenital Cytomegalovirus Infection. Pediatric Publications. Web.
Kimberlin, D. W., Jester, P. M., Sánchez, P. J., Ahmed, A., Arav-Boger, R., Michaels, M. G., …, Han, J. Y. (2015). Valganciclovir for symptomatic congenital cytomegalovirus disease. New England Journal of Medicine, 372(10), 933–943.
Kim, Jik, B., Joon, J., Shin, Han, S., Kim, … Yoon, B. (2018, August 30). Characterization of Detailed Audiological Features of Cytomegalovirus Infection: A Composite Cohort Study from Groups with Distinct Demographics. Web.
Kimberlin, M.D., Penelope, M., Jester, B.S.N., Pablo J., Sánchez, M.D., Ahmed, A.,…, Sood, M. et al. (2015). Valganciclovir for symptomatic congenital cytomegalovirus disease. The New England Journal of Medicine, 372, 933-943.
Lazzarotto, T., Guerra, B., Gabrielli, L., Lanari, M., & Landini, M. (2011). Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clinical Microbiology and Infection, 17(9), 1285-1293.
Mack, Ines, Burckhardt, Marie-Anne, Heininger, Ulrich, … Sven. (2017). Symptomatic Congenital Cytomegalovirus Infection in Children of Seropositive Women. Web.
Manicklal, S., Emery, V. C., Lazzarotto, T., Boppana, S. B., & Gupta, R. K. (2013). Vol 26: 86-102 The “Silent” Global Burden of Congenital Cytomegalovirus. Web.
Marsico, C., & Kimberlin, D. W. (2017). Congenital Cytomegalovirus infection: advances and challenges in diagnosis, prevention and treatment. Italian Journal of Pediatrics, 43, 38.
Medela (n.d.). Welcome to Medela! 2020. Web.
Misono, S., Sie, K., Weiss, N., et al. (2011). Congenital Cytomegalovirus Infection in Pediatric Hearing Loss. JAMA, 137(1), 47-53.
Rawlinson,W. D., Boppana, S. B., Fowler, K. B., Kimberlin, D. W. et al. (2017). Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infectious Diseases. Web.
Revello, M., & Gerna, G. (2002). Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clinical Microbiology Reviews, 10, 680-715.
Revello, M. G., Tibaldi, C., Masuelli, G., Frisina, V., Sacchi, A., Furione. M., …, CCPE Study Group (2015). Prevention of primary cytomegalovirus infection in pregnancy. EbioMedicine, 2(9), 1205-1210.
Toriyabe, K., Morikawa, F., & Minematsu, T. (2017). Anti- cytomegalovirus immunoglobulin M titer for congenital infection in first-trimester pregnancy with primary infection: a multicenter prospective cohort study. Journal of Perinatology, 12, DOI: 10.1038 / jp.2017.133.
Walter, S., Atkinson, C., Sharland, M., Rice P., Raglan, E., Emery, V.C. et al. (2008). Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Archives of Disease in Childhood Fetal and Neonatal Edition, 280–285.
Yamamoto, A.Y., Mussi-Pinhata, M. M., Marin, L. J., Brito, R. M., Oliveira, P. F., Coelho, T. B. (2006). Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? Journal of Clinical Virology, 6, 228-230.