Summary
Thermogravimetric analysis (TGA) is a method of thermal evaluation of substances where deviations in physical and chemical properties of materials are quantified with changes in time or temperature. The purpose of this experiment was to use TGA to determine compositional information about an inorganic salt and formulate a series of experiments to enable the analysis of a rubber sample. About 16.3 mg of NiCl2.xH2O and 26.6 mg of rubber were analyzed in a TGA50 analyzer separately according to the manufacturer’s operating procedure. NiCl2.xH2O dehydrated within the expected temperature range of 400oC and 600oC.
Thermal decomposition of the rubber sample produced three components: oil at 300oC to 400oC, a polymer at 400oC to 450oC, and carbon black at 500oC to 600oC. The sequence of separations corroborated literature findings. Experimental errors such as incorrect measurements of temperature and time affected the agreement between theoretical and experimental waters of hydration values for the inorganic salt. Overall, it was concluded that TGA was a reliable method in the determination of the chemical composition of materials. Future experiments should consider using complementary methods to evaluate the rubber sample to facilitate precise reverse engineering of the polymer.
Introduction
Chemical substances undergo various changes when heated. These changes can be analyzed and used to deduce valuable information about the substance. Thermogravimetry (TG) is a division of thermal analysis that investigates the changes in masses of substances with respect to temperature (scanning mode) or in connection with time (isothermal mode).1 Thermogravimetric analysis (TGA) is a system of thermal assessment where fluctuations in physical and chemical attributes of substances are quantified as a function of temperature or time. Variation in mass is attributed to a number of thermal occurrences such as absorption, oxidation, desorption, vaporization, sublimation, reduction, and disintegration. These processes are investigated as the sample is exposed to a predetermined schedule of temperature change. Consequently, TGA is used in the investigation of volatile substances and gaseous liberated during reactions involving elastomers, thermoplastics, paints, composites, thermosets, films, coatings, and fibers, among others.2
Three types of TGA are possible: isothermal or static, quasi-static, and dynamic TGA. In static TGA, the sample is held at a steady temperature for a specified period during which alterations in weight are documented. Conversely, the quasi-static approach entails heating the test sample to an invariable weight at various series of increasing temperatures. Dynamic TGA a stable heating rate is used to determine the increase in temperature with respect to time.
TGA has several applications, including finding the temperature versus weight alterations in decomposition reactions, thus enabling quantitative constitution analysis. The water content or residual solvent in a substance can be determined using this technique. Additionally, it is possible to determine how chemical substances react with oxygen, air, and any other reactive gas using TGA. The rate of evaporation with respect to temperature, for instance, the volatile discharges of liquid combinations can be quantified via TGA. Similarly, through TGA, the purity of materials, as well as the identity of organic substances, can be ascertained.2
The purpose of this experiment was to determine compositional information about an inorganic salt using thermogravimetric analysis and find out the temperature conditions under which the salt dehydrated. Another goal was to devise a series of experiments that would permit the analysis of a rubber sample. It was hypothesized that the waters of hydration of the inorganic salt would be successfully determined using a thermogravimetric analyzer.
Methods
One preliminary thermogravimetry experiment was conducted to determine the water of hydration of nickel chloride hydrate before evaluating the rubber (EPDM) sample. This choice was informed by the need to use a known compound to ascertain the efficacy and reliability of the TGA50 analyzer before subjecting the test sample of interest to the same procedure. A continuous flow of nitrogen gas was provided in the TGA50 analyzer before commencing the experiment. Approximately 16.3 mg of the salt was weighed using an analytical balance and placed onto the sample pan. The instrument was operated according to the manufacturer’s operating procedure and used to measure changes in the mass of the salt at various temperatures in intervals of 1 second. A similar procedure was repeated using 26.6 mg of the EPDM sample. Changes in mass with increases in temperature were recorded. The constituents of EPDM at different temperatures and time intervals were also recorded.
Results
Figures 1 and 2 illustrate representative plots of temperature versus mass loss for NiCl2.xH2O and EPDM respectively. Table 1 shows the observed mass losses for dehydration steps of NiCl2.xH2O in flowing argon, whereas Table 2 shows the observed mass losses for degradation steps of polymer EPDM in dry air. The experimental water lost for EPDM was compared to the literature and summarized in Table 3.
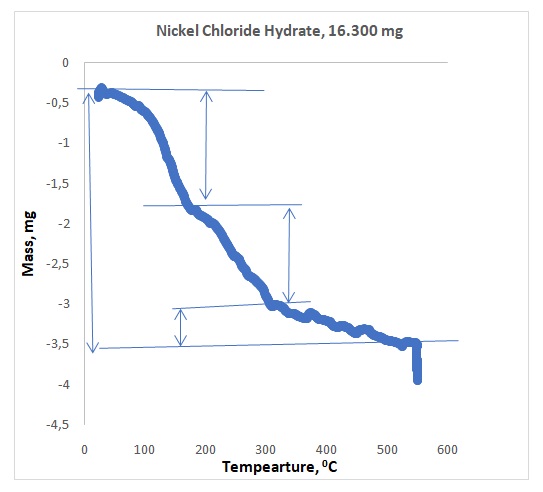
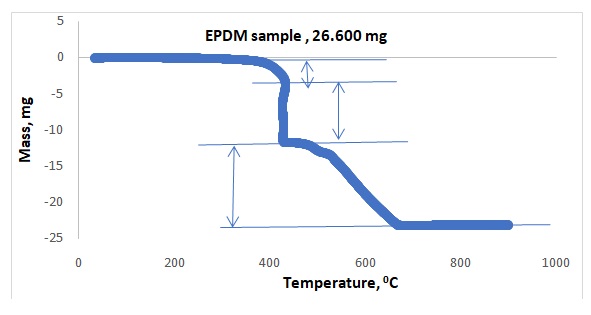
Table 1. Observed mass losses for dehydration steps of NiCl2.xH2O in flowing argon.
Table 2. Observed mass losses for degradation steps of polymer EPDM in dry air.
Table 3. Experimental water lost for NiCl2.xH2O at different stages versus literature values.
Preliminary research showed that the compound NiCl2.xH2O dehydrated between 400 and 600oC, which was the expected temperature range. Actual dehydration took place between 500 and 600oC. All the waters did not come off at once because 1.465 mg of water came off within 100oC to 200oC, 1.205 mg came off within 200oC to 300oC, and 0.496 mg came off within 500oC – 600oC. All the waters did not come off the crystal because, in Figure 1, the plateau of the graph showing the presence of water at 500oC -600oC. The rubber sample provided three components. The first component that came out was oil at 300oC – 400oC, whereas the second component was the polymer at 400oC– 450oC, and the last component was carbon black that came out at 500oC – 600oC. The sequence of separations agreed with the literature value.4
The findings of thermogravimetry can be complemented with data from microscopy, X-ray photoelectron spectroscopy (XPS), and Fourier-transform infrared spectroscopy (FTIR). Microscopy entails scrutinizing minute structures that are invisible to the naked eye using specialized equipment. XPS is a quantitative spectroscopic method that is highly sensitive to surface characteristics. It quantifies the composition of different chemical elements in a substance at the parts per thousand scale. The data generated by this technique can be used to compute the empirical formula of a substance as well as to determine the chemical and electronic states of the elements found within a material.5 Conversely, FTIR is an analytical method that makes use of infrared light to scan samples and scrutinize their chemical attributes thus aiding in the identification of organic, polymeric, and inorganic materials.6 During the process, an absorption or emission infrared spectrum of the test sample is generated. FTIR is commonly used in applications such as food analysis, polymer science, organic synthesis, petrochemical engineering, and pharmaceutical production. Given the time limitation, microscopy is a complementary method that could have been used beforehand to give an idea of the expected findings following thermogravimetric analysis.
Comparing the findings of the preliminary experiment using the known inorganic salt with the literature values showed some discrepancies, which could be representative of the error component of the TGA50 system. It was likely that these errors were replicated in the thermogravimetric analysis of rubber. Known sources of error in similar experiments include the measurement of temperature and mass in addition to inaccuracies in the calibration of the instrument. Buoyancy is reported to alter the precision of TGA findings.7 This error can be accounted for by conducting a blank measurement and subtracting its value from the actual sample measurement. Inconsistencies in sample preparation can also affect the findings. For example, a chemical substance could change its composition due to stress, transport, or storage conditions, which could ultimately influence the findings obtained during TGA. Any contamination of the sample before or during the experiment could also alter its TGA outcomes and provide a false representation of its chemical composition.
Conclusion and Recommendation
Through this experiment, it was possible to determine the waters of hydration of NiCl2.xH2O as well as the chemical composition of rubber using TGA within a limited time. The constituents of rubber obtained experimentally matched the reported components of rubber. However, there were discrepancies in the experimental and theoretical values of waters of hydration in the inorganic salt, which pointed towards possible sources of error in the procedure. These included inaccuracies in measuring the samples, calibration of the instrument, and likely contamination. It was concluded that TGA was a reliable analytical method of determining the chemical composition of substances. Future studies should consider addressing the sources of error to enhance the accuracy of the results. Furthermore, sufficient time should be given to permit the implementation of additional complementary analyses to ascertain the chemical composition and proportions of each constituent. Such analyses could pave the way for the accurate reverse engineering of the product.
References
Yao, Z.; Ma, X.; Wang, Z.; Chen, L. Characteristics of Co-combustion and Kinetic Study on Hydrochar With Oil Shale: A Thermogravimetric Analysis. Appl. Therm. Eng. 2017, 110, 1420-1427.
Gaisford, S.; Kett, V.; Haines, P. editors. Principles of Thermal Analysis and Calorimetry; Royal Society of Chemistry: Cambridge, 2016.
Mishra, S.; Kanungo, S. Thermal Dehydration, and Decomposition of Nickel Chloride Hydrate (NiCl2· x H2O). J. Therm. Anal. Calorim. 1992, 38, 2417-2436.
Kwon, E.; Castaldi, M.J. Investigation of Thermo-Gravimetric Analysis (TGA) on Waste Tires and Chemical Analysis Including Light Hydrocarbons, Substituted Aromatics, and Polycyclic Aromatic Hydrocarbon (PAH). 15th Annual North American Waste-to-Energy Conference, 2007, 183-190.
Liu, J.; Ma, J.; Luo, L.; Zhang, H.; Jiang, X. Pyrolysis of Superfine Pulverized Coal. Part 5. Thermogravimetric Analysis. Energy Convers. Manag. 2017, 154, 491-502.
Qi, Y.; Wu, W.; Han, L.; Qu, H.; Han, X; Wang, A.; Xu, J. Using TG-FTIR and XPS to Understand Thermal Degradation and Flame-Retardant Mechanism of Flexible Poly (Vinyl Chloride) Filled with Metallic Ferrites. J. Therm. Anal. Calorim. 2016, 123, 1263-1271.
Balme, Q.; Lemont, F.; Rousset, F.; Sedan, J.; Charvin, P.; Bondroit, J.; Marias, F. Design, Calibration and Testing of a New Macro-Thermogravimetric Analyzer. J. Therm. Anal. Calorim. 2018, 132, 1439-1447.