Brief History
Basically, a battery is comprised of numerous electrochemical cells. In 1792, an Italian physicist by the name of Alessandro Volta came up with the first electrochemical cell. By 1800, he had developed the first battery comprising of multiple electrochemical cells. It was Luigi Galvani’s discovery that spurred Volta’s desire to develop a battery. In 1870, Galvani discovered that when frog’s legs came into direct contact with a spark they twitched. He further discovered in 1786 that the twitching could also take place during lightning (Linden & Reddy pp. 214-123). A year after, Volta had learned the processes that take place in capacitance helping him to come up with an electric circuit consisting of cardboards saturated with salt water and other electric detectors.
In 1800, Volta discovered that he could come up with a battery by arranging a group of voltaic cells in series. This arrangement helped Volta increase the amount of electromotive force produced by the cells.
Despite the batteries being highly valued in supporting experiments, they did not offer sufficient voltage due to fluctuations. It was hard for the batteries to support experiments that took a long time to complete. Continued efforts to develop more reliable batteries led to the discovery of the Daniell cell in 1836. The battery manufactured from these cells was reliable and offered sufficient voltage thus being used by industries in running static machines. The cells contained liquid electrolytes that were susceptible to spillage. In addition, the batteries were made of glass containers thus making them more delicate. It is these features of the battery that made it hard for it to be used in running mobile machines (Linden & Reddy 125). At the end of the nineteenth century, a dry cell battery was developed making it possible for the battery to be used in mobile machines. After this invention, the battery gained popularity and is currently being used in numerous fields.
How a battery works
For a battery to emit electric energy, the chemical energy stored in the battery is transformed into electrical energy. As aforementioned, a battery is manufactured from a collection of voltaic cells. Every cell consists of two half cells that are sequentially linked by using a conductive electrolyte. The electrolyte contains cations and anions. One of the half cells is made up of electrodes and electrolytes towards which cations drift towards. This electrode is termed the cathode. On the other hand, the other half cell contains electrodes and electrolytes to which anions drift towards. This is the negative electrode is termed as the anode. The battery is powered through a redox reaction where electrons are added to the anode while at the same time removed from the cathode. Anode and cathode are not in direct contact and are connected through the electrolyte (Battery Council International para. 2). There are batteries that are made of two half cells with each of the half cells using a unique electrolyte. The half cells are thus separated using a separator to prevent the two electrolytes from mixing.
When in use, a lead-acid battery undergoes a continuous process of charging and discharging. When connected to an electric appliance, current flows from the battery to the appliance thus discharging it. On the other hand, if a battery is connected to a car and no accessories are using it, the vehicle’s alternator recharges the battery. During the discharge process, the electrodes are found to be chemically similar, the voltage decreases and the acid becomes weaker. If the process continues for a long time, the battery becomes weaker to an extent that it can not discharge valuable electricity (Battery Council International para. 3-5). The figures below describe the charging and discharging process in lead-acid batteries.
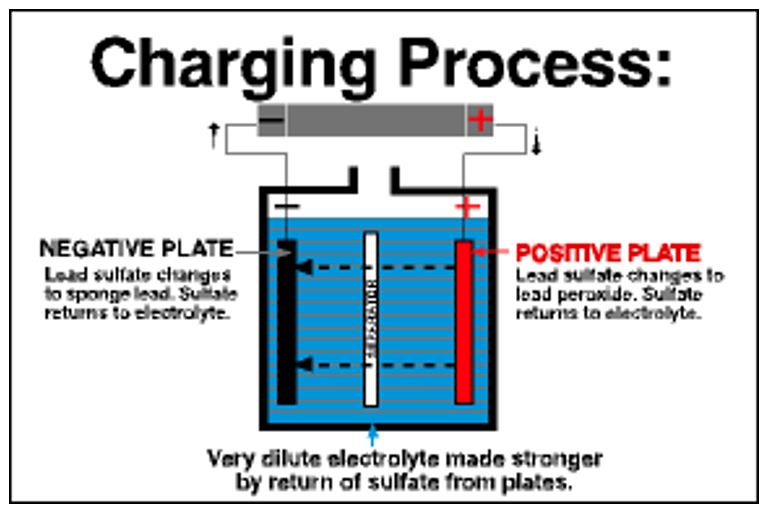
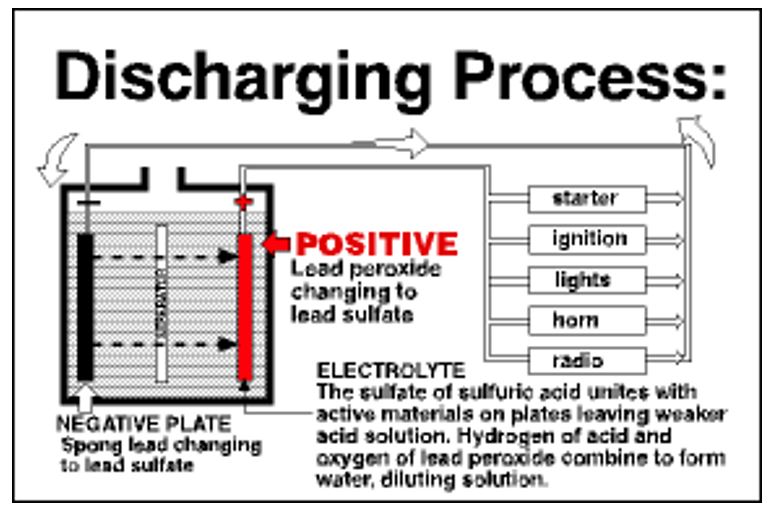
Why the battery is heavy and stores a small amount of energy
The main reason behind the heavyweight of the battery is the materials used in manufacturing it. For instance, lead-acid batteries contain the acid that acts as the electrolyte and the lead electrodes. These materials are heavy adding to the weight of the battery. Even the materials used in manufacturing dry cell batteries contribute to the weight of the batteries.
The amount of energy stored by a battery depends on the amount of the electrolyte and electrode contained in the battery. It is difficult to manufacture a battery with more electrolytes and electrodes and at the same time cut down on the size of the battery. Consequently, the manufactured batteries store a limited amount of energy. There is a continuous chemical reaction in a battery. Factors such as the permissible terminal voltage, temperature and amount of current in a battery make it hard for a battery to store a huge amount of energy (Linden & Reddy 137). They are referred to as the discharge conditions. To ensure that a battery stores a huge amount of energy, there is a need for manufacturers to increase the amount of electrolyte as well as the size of the electrode plates used.
Energy crisis being experienced
For decades, countries have depended on oil and other fossil fuels as the main sources of energy. As more industries and equipment powered by energy from these sources crop up, the depletion rate of the sources of energy has increased. Consequently, oil fields and fields that supply other fossil fuels such as coal are getting exhausted. This implies that if no other sources of energy are invented in the near future, most of the world’s industries are going to come to a standstill due to a lack of energy to run them. With time oil prices in the world have continued going up. Unlike other commodities that have numerous substitutes emerging in the market, no “new” oil has been reported to come into the market (Deffeyes 134). Some of the oil-producing states claimed to have numerous oil reserves. Nevertheless, this can not be trusted as it has been witnessed in the past to be a strategy used by states to influence oil prices in the market. For instance, the United States claimed to have greater oil reserves in 1979 only to be unable to produce the required amount of oil leading to a reduction in the amount of oil production.
Apart from the oil shortage being experienced, oil and other fossil fuels are major contributors to environmental pollution. Their increased rate of usage has led to an intensification of global warming and other forms of environmental pollution. Currently, the world is vulnerable to a rise in sea level due to temperature resulting from global warming. Moreover, the volume of global food production is going down subjecting the world to hunger and starvation (Deffeyes 138). If countries do not look for alternative sources of energy, future generations will be faced with numerous challenges caused by global warming and the energy crisis.
Last achievements in the energy field
The call for environmental conservation and the need for alternative sources of energy to cater to the oil crisis has led to stakeholders in the energy and industrial sectors looking for alternative sources of energy as well as developing machines that rely on alternative sources of energy rather than oil and coal. One of the recent breakthroughs was the development of electric cars. These cars use varied sources of electric power ranging from electric motors to batteries. Besides, there has been the establishment of electric airplanes (Leitman & Brant 87). Today there are numerous manned and unmanned planes that are powered using electricity.
This discovery has helped in cutting down on environmental pollution. The vehicles are less noisy compared to those propelled by oils and emit a limited amount of carbon dioxide. Besides, it is possible for different countries to locally manufacture and use electricity. Thus the development has helped in energy resilience (Leitman & Brant 89).
In spite of the benefits accrued from using electric energy as the source of power for automobiles and industries, there are numerous demerits that accompany the use of this source of power. Most of the current batteries being used in electric vehicles, planes and other appliances can only store a limited amount of energy. The batteries used can not store a lot of energy compared to gasoline. Hence, it is difficult to keep the vehicles or machines running on electricity for a long time (Leitman & Brant 89). On the other hand, most of the current sources of electricity contribute to environmental pollution. For instance, America gets its electricity from fossil fuels thus not cutting down on the rate of environmental pollution.
Future of the battery industry
The future of the battery industry is promising. Hopes are high that in the near future high efficient batteries will be in the market. Experts are in the process of integrating two technologies of battery systems in one to come up with a more reliable battery. The lithium-ion batteries used in the motor vehicle industry are credited for discharging enough power for accelerating vehicles. Nevertheless, they are not reliable as they can not keep a vehicle running for a long time (Merfeld para. 4). On the other hand, batteries manufactured from sodium are capable of storing a lot of energy but they discharge a limited amount of power that can not accelerate a vehicle. Consequently, experts are looking for ways to incorporate the technologies used in manufacturing the two varieties of batteries into one technology. This will help them come up with light batteries capable of striking a balance between electric range and acceleration (Bullis para. 5). This will be used in running steam engines, industrial machines as well as mining trucks thus saving the world from the oil crisis and environmental pollution. With more airplanes using electric power to run their electronic systems, the development of these batteries will significantly help the aviation industry. As this initiative is being supported by the governments from different states, chances are high that the energy crisis will be history in the future.
Conclusion
Based on the rate at which oil deposits are getting exhausted, the new kind of batteries that will be capable of efficiently running heavy machines will facilitate in reducing the oil consumption rate. The batteries being manufactured will be capable of running machines efficiently for a long time. In addition, the technology being used in manufacturing these batteries is environmentally friendly. Hence, the implementation of the new batteries will help in controlling the rate of environmental pollution. The batteries emit a limited amount of carbon dioxide so they will help in cutting down on the rate of global warming.
Works Cited
Battery Council International. “How a battery works.” 2010. Web.
Bullis, Kevin. “A guide to recent battery advances.” Web.
Deffeyes, Kenneth S. Hubbert’s Peak: The Impending World Oil Shortage. New Jersey: Princeton University Press, 2003.
Leitman, Seth & Brant, Bob. Build Your Own Electric Vehicle, 2nd Edition. New York: McGraw-Hill, Inc., 2008.
Linden, David & Reddy, Thomas B. Handbook of Batteries. New York: McGraw-Hill, 2001.
Merfeld, Glen. “A new generation of batteries awaits.” Environmental Leader. 2010. Web.