Flow batteries also known as Redox batteries have been in existence since 1880s and zinc/chloride the first kind of the batteries was first used by Charles Renard in 1884 to power his airship called La France (“Battery and Energy Technologies”). In flow batteries, current is produced in the process of electrochemical reduction of one type of electrolyte while the others type is oxidized in the similar way. This is made possible when electrolyte is driven from one tank to the other across the membrane that separates the two tanks. Reaction in the setup is irreversible and as a result, current is generated and collected in electrodes ready for consumption. The process enables recharging, discharging and charging of batteries as shown in the following figure below (see fig 1).
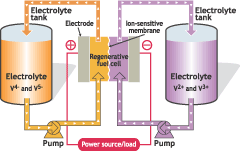
The batteries have two paramount advantages in that they have exceptionally long or limitless life and they are relatively cheap compared to other energy sources. There are four main different designs of flow batteries, which include iron Chromium flow battery, zinc bromine flow battery, vanadium flow battery and zinc chloride flow battery.
Zinc Bromine Flow Battery which is a mixture in nature and rather more fixed than the other flow batteries is limited in energy production. This is due to the fact that during the charging process zinc metal gets plated on the anode implying that the more the surface area available for plating, the more energy will be produced. (“Flow Batteries”). Since Zinc Bromine cells remain in the ionized solution when discharging, the flow battery cannot suffer the effect of different reactants. The flow battery uses cheaper and readily available reactants. The trouble with Zinc Bromine flow battery is that during Zinc plating process, dendrites growth is realized and it also produces Bromine gas which is toxic. Zinc Bromine Flow Battery is ready for use on a broad scale as it is presently being produced as much as 500KW/2.8MWh on a single package.
Iron Chromium Flow Battery which was first developed for Apollo program has different reactants on the anolyte and the catholyte which are FeCl3 and CrCI2 respectively. The two reactants pose risk to the battery since it can lead to permanent loss of the battery’s capacity. This is possible during the transfer of ions across the membrane especially when the membrane is either damaged or inefficient. In addition, in order to produce sufficient energy, bigger electrolyte tanks are required due to iron chromium low energy density (“Flow Batteries”). The Flow Battery is the safest flow battery and it uses comparatively less harmful chemicals. The Battery is not used on broad scale but is currently being developed and redeveloped for utility applications.
Vanadium Flow Battery according to “Flow Batteries” uses one reactant on anolyte and catholyte cells hence it has no problem of ionic transfer as is the case in Iron Chromium. The Vanadium reactants are higher in cost compared to iron chromium and Zinc Bromine and it has also safety risks as one of its reactants, PentaOxide powder is very dangerous. Even though the Flow Battery has low energy density, it is considered the most efficient of all the flow batteries. The flow Battery is produced in a broad scale for asset transmission even to the tune of 500KW/2MWh. The flow battery has limitless cycles of between 10 to 15 years.
Zinc Chloride Flow Battery was first developed between 1970 and 1990 by a company called Energy Development Associates (EDA). Unfortunately the technology could not flourish then due to lack of potential market which led to its expiry. However, currently the technology is under serious redevelopment and development by some startups thus the product is not in use on a broad scale at present (“Flow Batteries.”).
Flow batteries are in this era among the least expensive sources of energies for utility scale batteries that have started picking up and are being tested for utility application and some manufacturers are already providing utility scale batteries while others are busy venturing into the industry.
Works Cited
“Battery and Energy Technologies.” Electropedia. 2005. Web.
“Flow Batteries.” Silent Energy – LLC. 2009. Web.
Kuntz, Mark., and Dawe Justin. Renewable.Rechargeable. Remarkable. 2005. Web.