Introduction
Seventy percent of the earth is covered by water or oceans. A large number of pollutants by way of land runoff and direct discharge are hurled into the ocean waters (Cui et al, 2008). The sediments could be having crude oil or a mixed group of polycyclic aromatic hydrocarbons or PAHs. Incomplete combustion of fuels having carbon and naturally occurring coal and crude oil contributes to the PAHs. These are consumed by organic material and sediment particles (Albers, 2003).The accumulation of pollutants has raised the risk of a gradual transition in the neighboring environments. Even remote deep-sea areas which are oligotrophic are contaminated with man-made pollutants (Cui et al, 2008). The marine pollutants are mainly polycyclic aromatic hydrocarbons (PAHs).
Polycyclic aromatic hydrocarbons (PAHs) constitute one of the largest groups of compounds that produce widespread organic environmental pollution posing a risk to marine biota (Goncalves et al, 2008). They are biodegraded by bacteria in deep-sea environments below the surface. This is evidenced by the sediments found 2.2m. under the bottom surface about 3542 m. below the water surface (Cui et al, 2008). The PAHs are there through the accumulation by deep-sea organisms (Porte et al, 2000). They are formed by abiotic and biogenic processes in vent systems (Proskurowski et al, 2008). Their combined actions on aquatic animals are still only remotely understood. The responses of fish to these compounds are investigated in experiments; their locomotor activities and social behaviors are studied (Goncalves et al, 2008). The differences between predicted and observed behaviors are noted.
PAHs exist as complex mixtures rather than single chemicals. Assessing combined toxicity is a problem as combinations can vary from region to region. Modeling approaches that predict the actions of the biota of the aquatic region based on the individual actions of the single chemicals appear to be better.
Aquatic toxicology accepts “concentration addition” (CA) as the quantitative estimation for the joint toxicity (Goncalves et al, 2008). A recent study indicated that there is a tendency to “overpredict” the joint toxicity using CA (Olmstead and LeBlanc, 2005). The CA works for the crustacean amphipods (Landrum et al, 2003) but its use in fish and endpoints like social behavior is questioned. Moreover in fish, 2 or more modes of action of the PAHs are possible: a non-specific narcosis, an interaction with the aryl hydrocarbon receptor or other mechanisms (Barren et al, 2004). Whether CA is sufficient to study the joint toxic effects has to be decided.
The influence of polycyclic aromatic hydrocarbons on embryogenic tissue of fish has been studied. It has been found that chronic exposure could produce deleterious effects on fish population size and growth (Heintz, 2007). The effects of reduced survival of 50% to maturity in pink salmon populations raise the possibility that some species would become extinct: 70 years of observation have by the Alaska Fisheries Science Center has reached the conclusion that aqueous PAH concentration of 18nL/L. produces “80 % decrease in population productivity and 11% probability of extinction after 35 generations” (Heintz, 2007).
Powerboating, water skiing and jet skiing are the motor recreational activities that have produced aquatoxicity (Mosisch and Arthington, 2001). Identifying the PAHs and estimating the quantity difference in the chemical contamination of Brown Lake in Australia, Mosisch pointed out the ill effects of recreational activities in the water. Mostafa (2002) indicated the amount of pollution in Lake Timsah in Egypt which has affected the seafood; carcinogenic PAH compounds indenopyrene, benzopyrene, dibenzoanthracene and the like were discovered. Pane and his colleagues (2005) discovered the carcinogenic hydrocarbon marine pollutants of the Ligurian Sea in the Western Mediterranean (Genova Gulf). Inputs were the organic pollutants from the land and the freshwaters (Pane et al, 2005).
Kim and Young indicated that the main inputs into the surface water in Sacramento River, California came from rain as 4-25% of colloid-associated PAHs while only 0.1-6% of the colloid PAHs came from surface water (2009). The PAHs of rainwater originated in pyrogenic sources while the surface water had diverse and complicated origins (Kim and Young, 2009).
Lang et al have studied the differences in the PAH concentration in different environmental situations in the Pearl River Delta, in China (2008). The higher molecular weight PAHs were found more at the site of emissions using actual emission rates. The PAHs in soil or sediment were found to have lesser of the lower molecular weight PAHs (Lang et al, 2008).
Mathew and his colleagues have indicated that the metabolism of the PAHs is occurring in the early life-stage fish (2008). This could be the explanation for the “discrepancies noted between the TLM (target lipid model) predictions and the measured toxic effect levels” (Mathew, 2008). A better measure could be the maximum body burden of parent PAH plus metabolites: this could also produce a “better comparison between the TLM predictions and measured effect levels” (Mathew, 2008). Understanding the critical body burden of the PAHs, the parent compound and its metabolites have to be studied.
Aging of the sediments or desorption is another factor that decides the bioavailability of the PAHs for uptake by the organisms (Moermond et al, 2007). The slow and very slow desorbing fractions do not affect the organisms. The fast desorbing fraction only is bioavailable. The food web accumulation could therefore be influenced.
Justification:
- Several studies suggest that polycyclic hydrocarbons derived from crude oil and coal are marine pollutants through the flow of rainwater and surface water from the land to the oceans. The marine biota is affected in population and size due to these pollutants.
- Aquatic toxicology studies need to investigate further how the carcinogenic PAHs can affect the fish and crustacean population which are consumed by human beings in all regions of the world and how much the marine population is really affected.
- Motor recreational activities are on the increase and this would further pollute large bodies of water and harm the marine animals which are human-consumed. Caution needs to be practiced if future generations are to consume healthy non-polluted seafood.
Objectives:
- Identify the PAHs which are the sources of marine pollution in the region by conducting a long-term study and highlight the methods by which they could be prevented.
- Determine if the type and quantity of PAHs identified in the region change annually for the worse. This can be compared to the incidence in other regions.
- Determine if the methods to prevent the pollution that can be caused by recreational activities can keep the pollution control.
Questions:
- What sources of marine pollution exist in the region? What are the PAHs found in the regions?
- Is the type and quantity of PAHs similar to the marine environment in other regions of the world?
- Can control of water recreational activities affect pollution?
Hypotheses:
- Sources of marine pollution will have a difference in the type of PAHs found in the marine world.
- The type and quantity of PAHs in the region will be influencing the extent of pollution.
- The water recreational activities cannot be controlled.
Methodology
The sources of the pollutants will be determined by acquiring the history and geography of the region and conducting field trips to specific sites in the region to discover the type of pollution that could occur: the rains, the surface water sources, any factories. Surface sediments from 70 sites in the first field trip and 55 midfield sites will be taken from different regions along the river and the delta region in the manner described in Clark Alexander’s study (Alexander et al, 2005). The target concentrations of the PAHs will be examined following the Model Toxics Control Act (Boyce and Garry, 2003). Risk-based concentrations, organic carbon partitioning in the water, biotransformation in the aquatic biota and distribution within the organism will be examined. The third hypothesis will be investigated through a structured questionnaire distributed among the visitors to the area and discussion with the local authorities who overlook the tourist activity in the region. Methods to reduce the pollution will be evolved in steps and shared with the authorities.
Data analyses
The physical characteristics or appropriateness for coring of the sediments will be examined using an X-ray machine. Each sample will be examined for characteristics
of obvious biological or physical mixing, sand content or overconsolidated character. Sediment accumulation rates will be geochronological determined. The type of PAH found will then be determined using coupled plasma mass spectrophotometry (Alexander et al, 2005). The questionnaires collected will be analyzed for the ideas of common people in terms of saving the marine population for posterity.
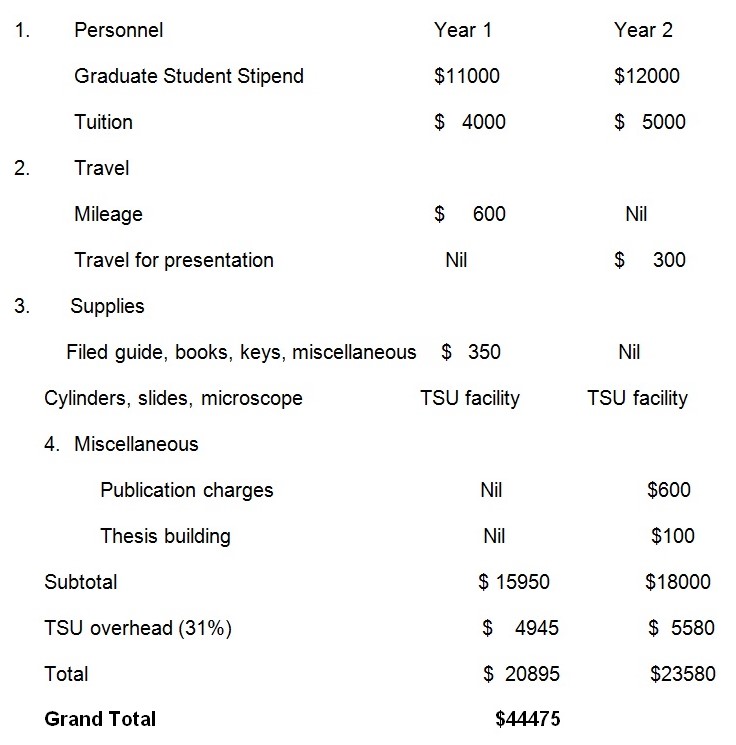
Budget
References
Albers, P.H. (2003). Petroleum and individual polycyclic aromatic hydrocarbons. In Hoffman D.J. et al (Eds.). Handbook of Ecotoxicology, Lewis Publishers, USA, p. 1-32.
Alexander, C.R., Lee, R.F., Burton, D.T. & Hall, L.W. (2005). An integrated case study for evaluating the impacts of an oil refinery effluent on aquatic biota in the Delaware River: Sediment core Studies. Human and ecological risk assessment, Vol.11, p.861-877, Taylor and Francis Inc.
Barron, M.G. et al (2004). Evaluation of fish early life toxicity models of chronic embryonic exposures to complex polycyclic aromatic hydrocarbon mixtures. Toxicological Science, Vol. 78, p. 60-67.
Boyce, C.P. & Garry, M.R. (2003 ). Developing risk-based target concentrations for Carcinogenic polycyclic aromatic hydrocarbons assuming human consumption of aquatic biota. Journal of Toxicology and Environmental Health, Part B. Vol. 6, p. 497-520, Taylor and Francis Inc.
Cui, Z., Lai, Q., Dong, C. & Shao, Z. (2008). Biodiversity of polycyclic aromatic hydrocarbon-degrading bacteria from deep-sea sediments of the Middle Atlantic Ridge, Environmental Microbiology, Vol. 10, Issue 8 p. 2138-2149.
Goncalves, R., Scholsze, M., Ferreira, A.M. Martins, Marta & Correia, A.D. (2008). The joint effect of polycyclic aromatic hydrocarbons on fish behavior. Environmental Research, Vol. 108, p. 205-213, Elsevier.
Heintz, R.A. (2007). Chronic exposure to polynuclear aromatic hydrocarbons in natal Habitats lead to decreased equilibrium size, growth and stability of pink salmon Populations. Integrated Environmental Assessment and Management, Vol. 3, No. 3, p. 351-363, SETAC.
Kim, D. & Young, T.M. (2009). Significance of indirect deposition on Wintertime PAH concentrations in an urban Northern California Creek. Environmental engineering Science, Vol. 26, No. 2, Mary Ann Liebert Inc.
Landrum, P.F., Lotufo, G.R., Gossiaux, D.C. Gedeon, M.L. Lee, J.H. (2003). Bioaccumulation and critical body residue of PAHs in the amphipod, Diporeia spp.: additional evidence to support toxicity additivity for PAH mixtures, Chemosphere, Vol. 51, p. 481-489.
Lang, C. Tao, S., Wang, X, Zhang, G. & Fu, J. (2008). Modeling polycyclic aromatic hydrocarbon composition profiles of sources and receptors in the Pearl River Delta, China. Environmental Toxicology and Chemistry, Vol. 27, No. 1, p. 4-9.
Mathew, R., McGrath, J.A. & Di Toro, D.M. (2008). Modeling polycyclic aromatic Hydrocarbon bioaccumulation and metabolism in time-variable early life stage exposures. Environmental Toxicology and Chemistry, Vol. 27, No. 7 p. 1515-1525.
Moermond, C.T.A. et al, (2007). Impact of polychlorinated biphenyl and polycyclic aromatic hydrocarbon sequestration in sediment on bioaccumulation in aquatic food webs, Environmental Toxicology and Chemistry, Vol. 26, No. 4, p. 607-615 SETAC.
Mosisch, T.D. & Arthington, A.H. (2001). Polycyclic aromatic hydrocarbon residues in the sediments of a dune lake as a result of powerboating. Lakes and Reservoirs: Research and Management, Vol. 6, p. 21-32.
Mostafa, G.A. (2002). Monitoring of polycyclic aromatic hydrocarbons in seafood from Lake Timsah. International Journal of Environmental Health Research, Vol. 12, p. 83-91.
Olmstead, A.W., LeBlanc, G.A. (2005). Joint action of polycyclic aromatic hydrocarbons: predictive modeling of sublethal toxicity, Aquatic Toxicology, Vol. 75, p. 253-262.
Pane, L. et al, (2005). Polycyclic aromatic hydrocarbons in water, seston, and copepods in a harbor area in the Western Mediterranean (Ligurian Sea). Marine Ecology, Vol. 26, p. 85-99 Blackwell Publishing Ltd.
Porte, C., Escartin, E., Garcia, L.M., Sole, L.M. and Albaiges, J. (2000), Xenobiotic metabolizing enzymes and anti-oxidant defences in deep sea fish: Relationship with contaminant body burden, Marine Ecological Program Services, Vol. 192, p. 259-266.
Proskurowski, G., Lilley, M.D., Seewald, J.S., Fruh-Green, G.L., Olson,E.J. & Lupton, J.E. et al, (2008). Abiogenic hydrocarbon production at lost city hydrothermal field, Science, Vol. 319, p. 604-607.