Zamra Health Center is an organization with a mechanism that ensures stability. There are systems that the internal stakeholder must adhere to in order to complete their designated duties. The organization may never succeed without managing some of its challenges both from within and outside. Risk management helps the firm to deal with eventualities that are sometimes beyond the standard procedures.
Quality Control Departments and Individuals
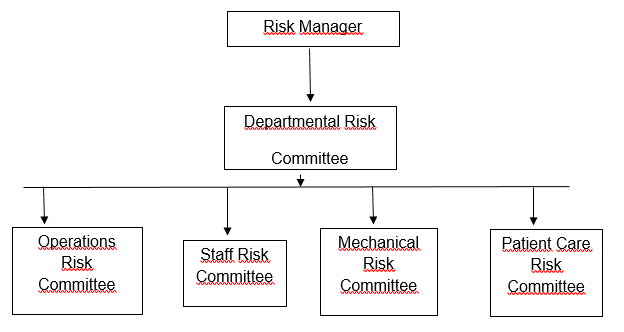
Risk deals with the possibility of an incident that might hamper the smooth running of the organization. The hospital has various departments dealing with specific functions. However, for control to be efficient the meeting established the risk management department (Agrawal, 2009). It will have multiple units. At the top is the Risk Management Supervisor. He should ensure that the affairs of this department run smoothly. He will also chair the risk committee meetings that typically take place every third week of the month.
Other sub-committees should typically meet on a weekly basis. Their decisions will culminate into the major resolutions at the risk committee meeting held on the third week of every month. All the chairpersons of the subcommittees must attend the monthly risk committee meetings. The subcommittees include departmental risk, the patient care risk, the staff risk, the operations risk, the mechanical risk, and the drugs and medicine risk committee (Warholak & Nau, 2010). Each member of the staff must report to his or her designated departmental supervisor.
Departmental risk committee will be taking care of the concerns in each risk department. It acts as the supervisory board for the other panels (Chorafas, 2013). The operations committee should address the needs of the hospital wards, payment systems, transactions, motivation challenges, and appreciations. It should also deal with the storage facilities, meals issues, and work processes.
The staff risk committee addresses the human resource concerns. It deals with the staff turnover, staff suggestions, disciplinary actions, appreciation methods and practices (Jordao & Sousa, 2010). The mechanical risk committee deals with the machinery, all software, and hardware in the organization. The patient care committee deals with all patient concerns. Lastly, the drug and medicine committee deals with the supply and use of the drugs and medicine.
Risk Management Regulatory and Accreditation Standards
Each department has risk management standards with detailed rules governing them. The staff risk department rules indicate that the department shall address all matters arising from individual employees and groups. The operations risk committee shall discuss any issues resulting from the activities and forward the resolutions to the departmental risk committee (Heller, 2009).
If the matters require in-depth skills and knowledge, then the departmental committee takes over for the final settlement. Mechanical risk committee shall always have the specialists concerned. Their resolutions are not final until the risk manager approves them after consultations. The patient care risk committee should comprise of the nurse administrator, representatives of ward, customer care, security department, outpatient department, and at least two physicians. The guidelines require that the committee can also invite any other person that may have concerns about the patient care (Warholak & Nau, 2010).
Departmental Meetings
Before any unit can hold a meeting, the risk committee heads should send a memo to all concerned parties and copy it to the risk manager. They should have agendas, date, time, and place of meeting. All groups should hold meetings in the conference halls available in the institution. The memo should indicate in which particular room the meeting will be taking place. Impromptu and emergency meetings may occur when there are situations that require immediate attention. Such meetings are supposed to address the crises at hand and ensure that an amicable solution is available within the shortest time possible (Vincent, 2010).
Any person having grievances should communicate the concern through the unit chairperson. If the matters involve the leadership of the committee, then the staff involved can write a note and place it in the suggestion box. Only the risk manager should open the suggestion box to read the concerns of the staff. However, other mechanisms include teams joining hands to write a signed request for a meeting to address a particular issue. They should send the request to the departmental leader and copy it to the risk manager.
Prevention and Recovery
Each department has well laid out guidelines for duties and work. The management appoints leaders to each unit to improve supervision and ensure coordination of activities. There is a job description manual for every worker (Heller, 2009). There are daily activities. Each employee logs into the system when he or she passes through the reception. When the employees are about to leave, they also sign off the system by filling in the information concerning that day’s work.
The system analyzes the report and gives a score for the day’s achievements. There is an IT team that regularly prepares the report and presents the company’s results to the management. If there are any delays and setbacks, the management advises the team leader to speak with the staff or officers concerned. The team encourages best achievers with bonuses.
Risk management provides the organization with critical information for continuous growth. Organizational members need to support it through their effort. Good results and success will only come when the stakeholders work toward growth.
References
Agrawal, R. (2009). Risk management. Jaipur, India: ABD Publishers.
Chorafas, D. (2013). Quality control applications. London, UK: Springer.
Heller, A. (2009). Dresden teamwork concept for medical high risk organizations. New York, NY: Nova Science.
Jordao, B. & Sousa, E. (2010). Risk management. New York, NY: Nova Science Publishers.
Vincent, C. (2010). Patient safety (2nd ed.). Chichester, UK: Wiley-Blackwell.
Warholak, T. & Nau, D. (2010). Quality and safety in pharmacy practice. New York, NY: McGraw-Hill Medical.