Abstract
The knowledge of physiochemical properties of binary mixtures of solvents is of great importance for learning intermolecular interactions. The aim of the research was to investigate the hypothesis that the activation energy of a substance depends on intermolecular forces that arise in this substance. To test this hypothesis, activation energies of pure water, acetone, toluene, and xylene have been experimentally determined using the viscosity-temperature function. The viscosities have been investigated using the Cannon-Fenske viscometer and simplified version of Poiseuille equation for fluid flow for water-acetone and toluene -xylene mixtures over the entire range of mole fractions at 295.35-328.15K. These systems have been evaluated in terms of ideality using calculated fluidities. Activation energies of water, acetone, toluene, and o-xylene have been calculated from the experimental results of viscosity measurements. Based on the theoretical and experimental fluidities, interactions between water and acetone molecules and toluene and o-xylene molecules have been discussed. It has been reiterated that the stronger the intermolecular forces of interactions arising between the molecules, the higher is the activation energy.
Introduction
The question to be investigated in the given research is whether there is a correlation between the type of intermolecular forces found in solvents and physicochemical properties of these solvents, in particular, activation energy. Also, the research was aimed at evaluating the ideality of the binary liquid systems by comparing theoretical and experimental fluidities. It is considered that for systems in which intermolecular forces are stronger, values of activation energies will be greater (Adamson, 2012). This is because the amount of energy needed to begin a chemical reaction will be greater.
Major types of intermolecular forces found between molecules of liquids consist of van der Waals forces (which include dipole-dipole interactions and London dispersion forces) and hydrogen bonds. Dipole-dipole interactions arise between permanent diploes of polar molecules (Atkins and Paula, 2014). Hydrogen bonds arise between the hydrogen of one molecule and oxygen, nitrogen, or fluorine of another molecule. London dispersion forces arise between non-polar molecules because of temporary fluctuations in their electronic distributions. The solubility of polar solvents in other polar solvents is explained by dipole-dipole interactions which are attractive for two different polar molecules. In contrast, if one molecule is non-polar, dipole-dipole interactions become repulsive; that is why non-polar solvents are non-soluble in polar solvents.
In the water-acetone system, there are three types of intermolecular forces. There are hydrogen bonds that are formed by the partial negative charge of oxygen of the one molecule and the partial positive charge of hydrogen of another molecule (Wypych, 2019). There are also strong dipole-dipole interactions between permanent dipoles of acetone molecules. Lastly, the oxygen atom of acetone interacts with the OH-group of water. So, there is a hydrogen bond between the partial negative charge of oxygen of acetone and the partial positive charge of hydrogen of water. In the system toluene-o-xylene, only weak van der Waals forces arise (Kuhn et al., 2009). It can be expected that the viscosities of these two systems will be different due to the different intermolecular forces, and so will be the activation energies. The strength of intermolecular forces increases in the following sequence: dispersion < dipole-dipole interaction < hydrogen bonding. Thus, activation energies are also going to increase in the same manner.
To determine activation energies of pure solvents, their viscosities need to be calculated. A Cannon-Fenske viscometer will be used for measuring the time required for the meniscus of a liquid to pass from point a to point b. The design of the viscometer fits the equation experimentally derived and formulated by the French physician and physiologist Poiseuille (1847):
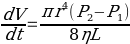
Poiseuille (1843) determined the relative viscosity of the sample tested by comparing it with the reference:
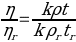
The relative viscosity of the sample tested can be calculated as:
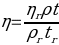
Theoretical fluidities of the systems and pure solvents will be calculated assuming that fluidities of ideal solutions are additive:
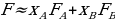 ,
,where XA and XB are molar fractions of components A and B of the mixture.
Experimental fluidities will be calculated using the formula:
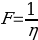
It can be expected that for ideal solutions, experimental and theoretical fluidities will be practically identical. The relationship between the temperature and viscosity was expressed by Arrhenius:
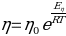
Methods
The experiment has been performed using the Cannon-Fenske viscometer that should be clean and dry. The solutions of water-acetone and toluene-o-xylene at different percent compositions by volume at 0, 20, 40, 60, 80, and 100% have been prepared. There have been the four parts of the experiment, each one performed at a different temperature (220C, 350C, 450C, and 550C). The temperature has been regulated by submerging the viscometer in the water bath. 8,00 mL of the liquid has been injected directly into the larger tube of the viscometer (Abbott-Lyon, 2019). During the experiment, the volume of liquid used and the flow times for the liquid solutions passing from point a to point b have been recorded to an accuracy of 0.001 s. Three sets of readings for the flow times have been taken, and their average has been used. It is necessary to be accurate at keeping the volume of a liquid constant and keep the pipette clean and dry. In the given experiment, water (t=250C) has been considered as a reference for all parts of the experiment.
Results
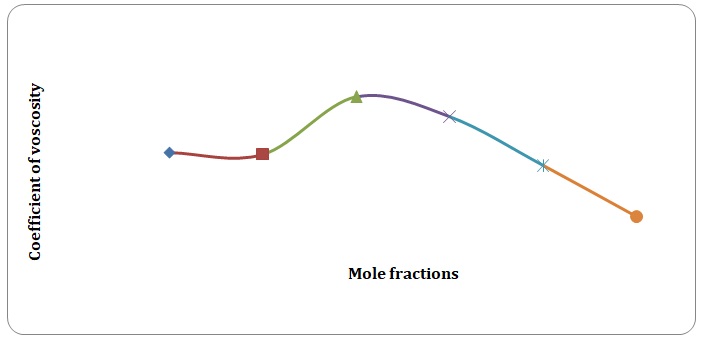
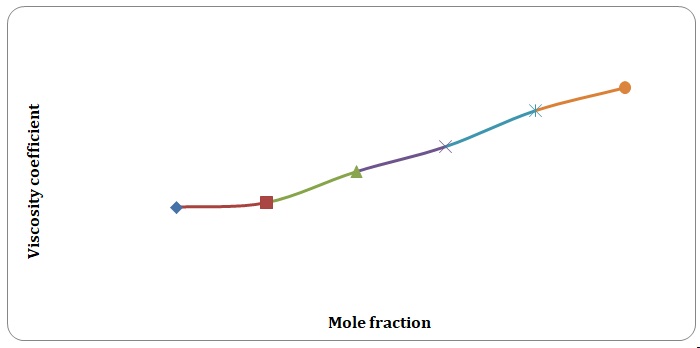
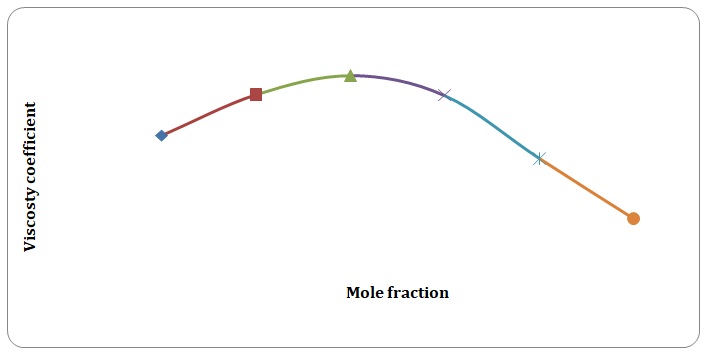
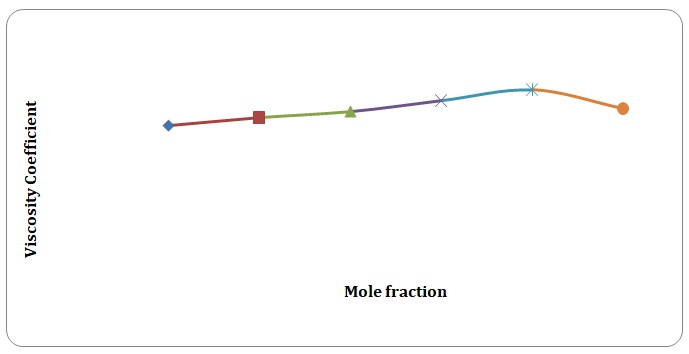
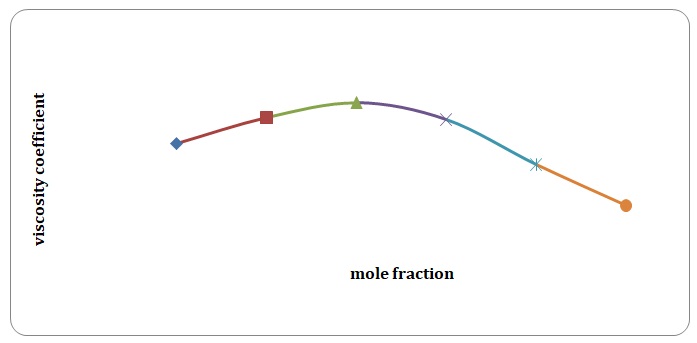
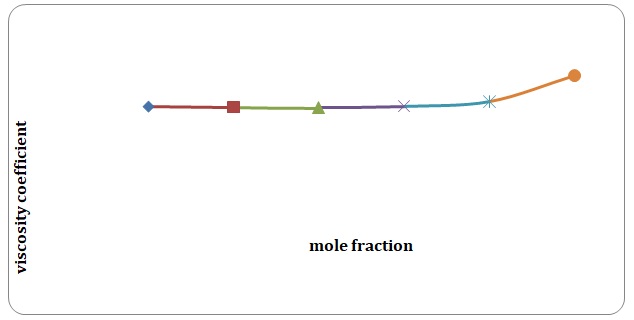
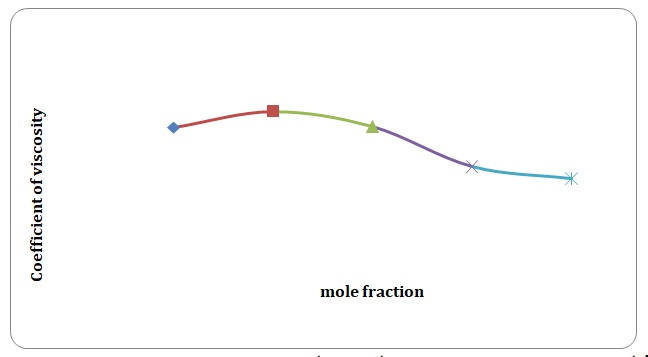
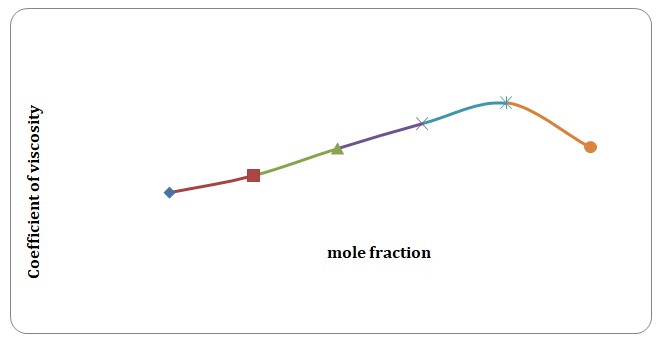
Based on the data obtained, the relative coefficients of viscosity for all pure solvents and mixtures have been calculated. Plots of the coefficients of viscosity versus mole fraction have been made (see Figures 1-8). It can be seen that it is possible to have a homogeneous binary liquid solution with a higher viscosity coefficient than that of both pure fluids making the solution (see Figures 1, 3, 5, 7).
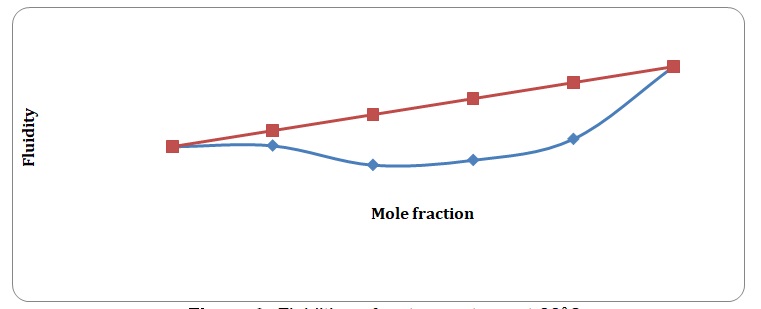
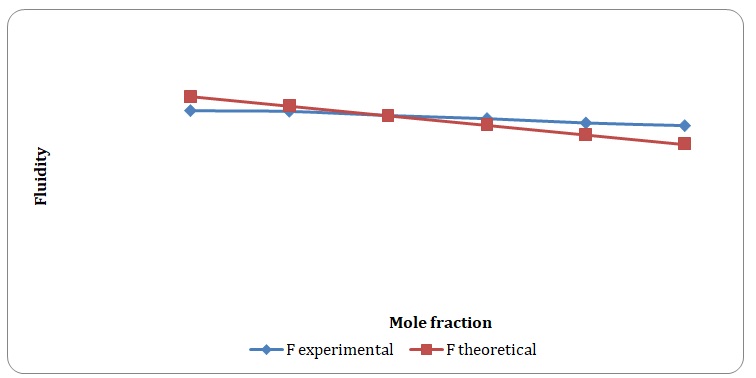
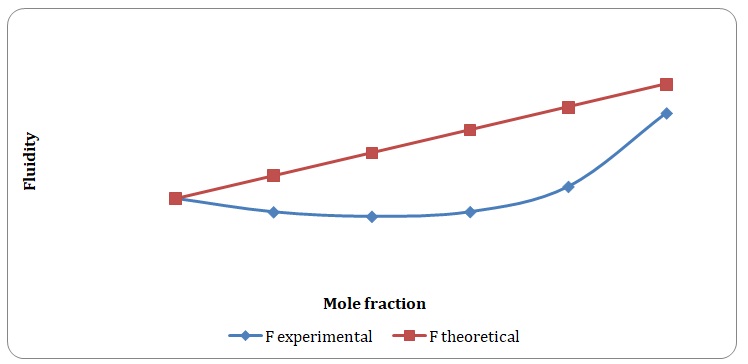
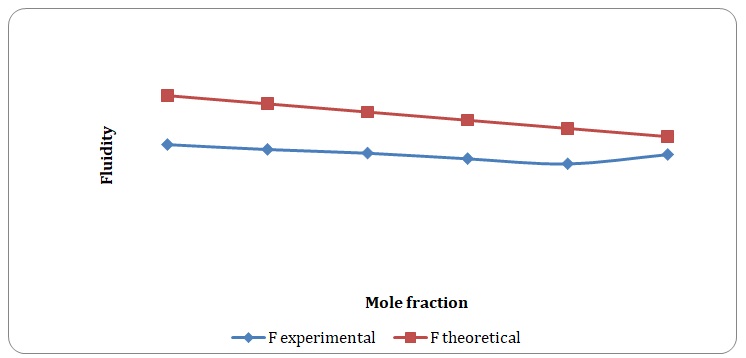
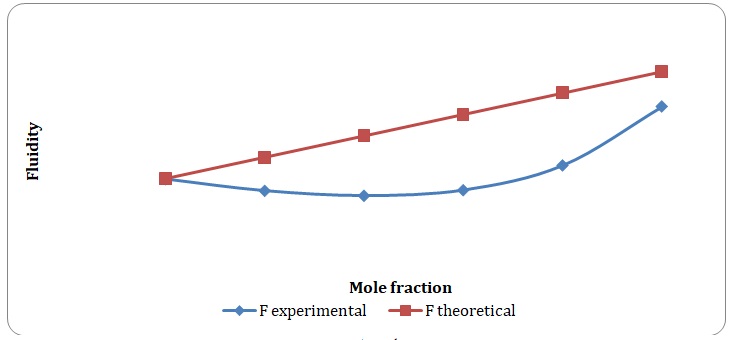
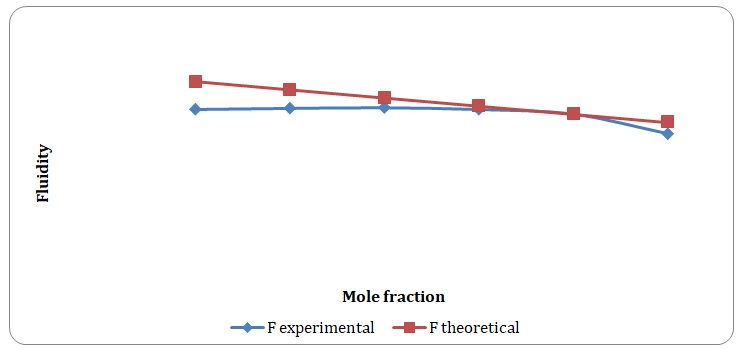
Theoretical and experimental fluidities have been calculated in order to determine the ideality of the water-acetone and toluene-xylene systems. For theoretical fluidities, inverse measures of theoretical viscosities of pure solvents have been used. Conclusions regarding the ideality of the systems studied have been made based on the differences between theoretical and experimental fluidities (see Figures 9-14). In the report, these differences will be further discussed and explained.
To determine the activation energy of pure solvents, the Arrhenius equation has been rearranged to the equation for a straight line:
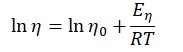
Graphs of ln η against 1/T have been created, and activation energy has been determined as a slope of a straight line.
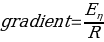
However, some of the data points have been rejected to keep the linear form of the plot (see Figures 15-18). Values of viscosities and so the values of ln η should decrease with an increase in temperature, though some data points do not fit the given tendency (see Table 1). This may be attributed to time measurement errors and the impossibility of measuring accurate results at high temperature.
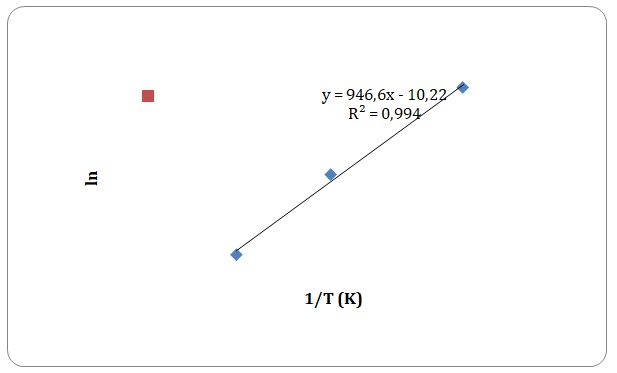
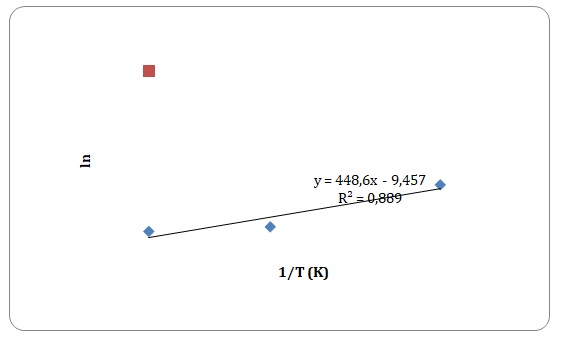
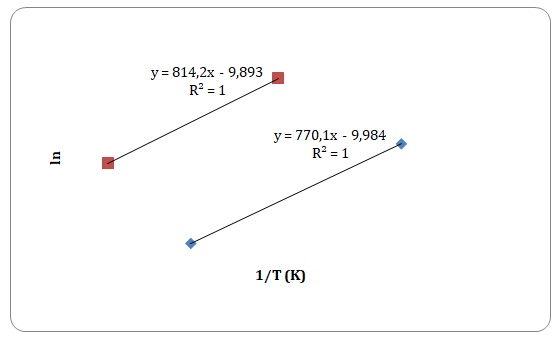
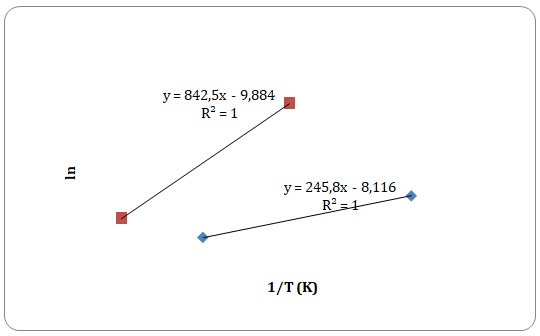
Table 1. Calculation of activation energies of pure solvents.
Discussion
It can be seen that the relative coefficient of viscosity of pure water is higher than that of acetone. This is due to strong hydrogen bonds that arise between water molecules and do not arise between molecules of acetone. High coefficients of the viscosity of water-acetone mixtures can be explained by strong dipole-dipole interaction between molecules of water and molecules of acetone. It can be observed that the higher the temperature, the lower is the relative coefficient of viscosity. As have been mentioned before, in the toluene-xylene system, only weak van der Waals forces arise. This is reiterated by comparatively low coefficients of viscosities. Based on viscosity coefficients, intermolecular forces between xylene molecules are stronger than those between toluene molecules.
There is a significant variation between theoretical and experimental fluidities of the water-acetone system, yet theoretical and experimental fluidities of the toluene-xylene system are almost the same. It may be assumed that the toluene-xylene system is close to the ideal solution. This means that intermolecular interactions that arise between molecules of toluene and xylene are almost the same as those intermolecular interactions that arise in the mixture of these solvents. In contrast, intermolecular interactions that arise in pure water and pure acetone differ from those that arise in the water-acetone system.
It has been stated that of all the four solvents, water is the one to have hydrogen bonds. Dipole-dipole interactions can be found both in water and acetone. Dispersion forces arise between molecules of all solutions, yet dispersion forces are stronger for xylene that has one extra carbon compared to toluene. Activation energy is directly related to the intermolecular forces of interactions that exist between the molecules. As was expected, the strength of experimentally determined activation energy increases in the following manner: toluene < xylene < water. The activation energy of acetone was expected to be higher than that of xylene and lower than that of water. However, the calculated activation energy of the acetone does not support the trend. This may be explained by experimental errors in measuring time flow.
Conclusions
Intermolecular forces condition a number of physicochemical properties of chemical substances. The viscosity of a mixture of solvents depends on intermolecular forces between the molecules of these solvents. Since viscosity is a thermally activated process, activation energy can be experimentally determined using experimental values of viscosity at the chosen range of temperatures. The stronger the intermolecular forces, the higher is the activation energy of a solvent. However, the accuracy of the given research is limited due to the measurement errors, which is why the activation energy of acetone did not fit the tendency.
References
Abbott-Lyon, H. Viscosity Lab Manual. Kennesaw State University: Georgia, 2019.
Adamson, A. A Textbook of Physical Chemistry, 2nd ed.; Elsevier: New York, 2012; pp. 280-294.
Atkins, P.; Paula, J. Atkins’ Physical Chemistry, 10th ed.; Oxford University Press: Oxford, 2014; pp. 838-840.
Kuhn, H.; Forsterling, H.; Waldeck, D. H. Principles of Physical Chemistry, 2nd ed.; John Wiley & Sons: Hoboken, 2009; pp. 835-845.
Poiseuille, J. L. M. The Flow of Liquids of Different Nature in Glass Tubes of Small Diameters. C. R. Acad. Sci. 1843, 16, 61-63.
Poiseuille, J. L. M. The Movement of Liquids of Different Nature in Tubes of Small Diameters. Ann. Chem. Phys. 1847, 21, 76-110.
Wypych, G. Handbook of Solvents: Use, Health, and Environment, 3rd ed.; Elsevier: Toronto, 2019; Vol. 2, pp. 1518-1530.