It is difficult to disagree with the suggested quotation because, in my understanding, it turns out to be absolutely correct. In order to justify my agreement with this opinion, it is necessary first of all to understand what exactly flowering plants, or, as they are often called, Angiospermae, are. Angiospermae are the highest forms of plants that have a flower — as the name implies — as a distinguishing feature. In other words, if a modern plant is capable of flowering, it is a sufficient sign to classify it to this clade. Flower in Angiospermae is formed as a result of modification of a shoot: in particular, such a shoot has limited growth and noticeably smaller size than a standard shoot in other plants. This shoot belongs to the reproductive system of flowering plants because, because of bud development, it contains stamens and pistils — these are male and female reproductive organs of plants, respectively. It is noteworthy that Angiospermae reproduce by seeds; the same form of reproduction is characteristic of Gymnosperm. However, unlike their evolutionarily older relatives, Angiospermae have flowers that protect the fruit during maturation. In addition, the flower attracts animals, which allows fertilizing plants and the spreading of seeds over long distances to expand the areola of Angiospermae. In other words, flowering plants are the most progressive form of plant life. Such plants are the most widespread plant group on the planet, and this fact is due to the ample opportunities for Angiospermae to interact with other forms of life.
In the proposed citation, particular emphasis is placed on the prevalence of Angiospermae on land. This is not surprising since, because of the evolutionary emergence of lower plants on land, there were vast expanses of unoccupied soil in front of the organisms. The lack of niche competitors, combined with evolutionary developmental mechanisms, led to the rapid evolution of lower plants into higher plants, and seeds became the dominant reproductive form to rapidly conquer vacant land. Angiospermae are thought to have appeared about 125 million years ago at the end of the Cretaceous Period. However, as plant genomics studies indicate, Angiospermae should not be considered as a daughter group of Gymnosperm but rather as a sister clade (Boundless, 2021). Consequently, there was intense competition for an ecological niche between the two clades of seed plants. The possibilities of embryo protection in flowering structures, together with the functions of double fertilization and the attraction of animals for reproduction, increased the evolutionary possibilities of Angiospermae for ecosystem predominance.
Other organisms play a unique role in such a strong distribution of Angiospermae on land. One of the best-known examples of this interaction is the symbiosis with insects that pollinate plant flowers. In particular, bees, butterflies, ants, bumblebees, and other insect species feed on flower nectar, which satisfies their biological need for nutrition. Bees are known to make honey from the nectar as recycled waste products to feed the next generations. At the same time, the insects get dirty in the pollen of plants and transfer this pollen from the anther c stigma — this is how the male Angiospermae seed is transferred to the female ovules. Consequently, the role of insects in pollination cannot be ignored. As a result, it allows plants to cross-pollinate and increase their ranges in the ecosystem. It is noteworthy that not only organisms participate in such pollination but also the wind. More specifically, some seed forms are adapted to planning, so air masses easily tear off such shoots and spread them over the ground, which also increases the likelihood of pollination.
Angiospermae, however, participate in relationships not only with insects but also with more advanced animal species, which include bats and birds. The beneficial effect of such animals on plants is not as unambiguous as in the case of insects. In particular, bats and birds also carry pollen between plants, which promotes the reproduction of plants. However, bats and birds also feed on the already ripened fruits of plants, which at first glance is an existential threat to Angiospermae. However, these animals consume the seeds along with the juicy fruit pulp, which are then spread throughout the ecosystem via the droppings. The droppings react as a helpful fertilizer, so new plants grow from the seeds.
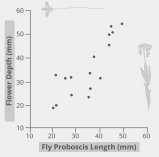
The biological cooperation mechanism described falls within the framework of co-evolution, a global developmental process in which some animal and plant species evolve together. The diagram shown in Figure 1 reflects the coevolutionary evidence for the relationship between insects and flowers. In particular, it has been demonstrated that the best co-evolution is characterized by pairs of organisms between which there are combining traits: the long proboscis of an insect combines with the long pollen tube of a plant, and vice versa. In other words, within a symbiotic relationship, Angiospermae develop such traits that are adapted to the animals of this ecosystem; this also works in the opposite direction. Moreover, it is this coevolutionary factor that is a predictor of such a wide distribution of Angiospermae in different ecosystems all over the planet.
It would be wrong to assume that the wide distribution of Angiospermae is the result of their cooperation only with animals. In fact, the flowers of Angiospermae may pollinate the flowers of other Angiospermae because of accidental pollen ingestion. This is an example of cross-pollination between related plant species of Angiospermae with a similar chromosome set. In this case, natural hybrids develop from the fruit, which combine traits of parental plants (Rhoades, 2021). As a result of long-term development of such hybrids, new Angiospermae species can be formed, which also increases their ecosystem superiority over other plant life forms.
Succession is an ecological phenomenon during which a successive change of biological communities occurs in a particular territorial area over time. The most classic example of succession is the emergence of a new plant community in place of a previously burned cover. The theory of succession was first developed by the American botanist Frederic Clements, who introduced the term “climax” into academic vocabulary in reference to successional processes (Knelman et al., 2018). Precisely, Clements understood climax as the comparably stable, stable existence of a biological community over time, with no changes due to the stability of internal processes. In most cases, however, the newly created community will not be similar to the one that existed in its place because ecological interactions are predictors of change.
Without the need to examine the factors that become predictors of the destruction of the parent plant community, it makes sense to consider the critical causes of successional change. In other words, it is essential to name the possible ecological interactions that can change successional processes and influence the development of daughter biological communities. The most obvious of such factors should include abiotic changes: climate, natural disasters, and weather conditions. If such factors influence the development of a daughter community, they cause a shift towards those forms of life which turn out to be adapted to successful existence in the altered environment. For example, desert cactus plants will not grow on land flooded by an anthropogenic factor. Another telling example would be a change in the acidity and mineral composition of the soil, rendering plants unable to live on that land; however, a change in pH may be favorable to other plants. In other words, abiotic factors are the primary predictor of any meaningful change during succession: they shape the topography and living conditions for new organisms.
In addition, the biotic factor should also be considered as a predictor of successional changes. When a daughter plant community is just beginning to develop, it attracts new animal species. The interaction of these pioneer animals with the environment leads to changes in succession. For example, if herbaceous Angiospermae grows in place of the earlier community, it attracts insects — flies, bees, ants — and birds, which increase the prevalence of this plant species in the community. The shift is especially noticeable during the first stages of the successional series when the rate of biomass growth is maximal.
Nor should one ignore the anthropogenic factor as making some of the fastest and most extensive changes during succession. When the parent community has been destroyed, humans can use the freed area for their own needs: urbanization, the development of agrocenoses with monocultures, or changes in the landscape. These changes directly affect how new species will survive in the transforming environment. For example, if a natural area has been used to build a city, it is unlikely that a forest community will grow on the site because humans regulate it. To put it another way, an anthropogenic factor makes essential changes to the successional process along with the abiotic and significantly alters the biodiversity of the daughter community. Eventually, almost any community will reach the climax when external factors cease to act on it.
One of the essential characteristics of successional succession is the different rates of biomass growth over time. Once the parent community has been destroyed, the ecological niche of the ecosystem is vacant and uninhabited. This creates favorable conditions for its rapid colonization by species of the daughter community. The plants are given the opportunity to reproduce almost unhindered in the area, which dramatically increases the biodiversity of the community. The attraction of symbiotic species also plays a role in expanding the diversity of plant species represented in the daughter community. Figure 2 shows this rate as a dramatic increase in biodiversity for a particular plant community ecosystem.
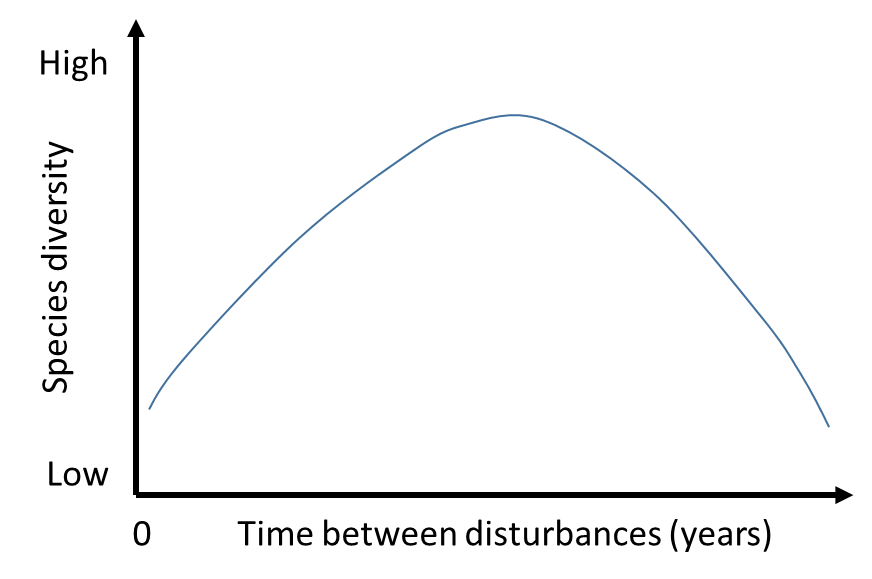
However, over time, the diversity in a plant community decreases rapidly, as shown in Figure 2. The reason for this decline is the development of a stable subsidiary community in which competition for resources, whether soil, water, or light, increases between species. Free, uninhabited territories and no limiting factors no longer exist, so plants have to compete with each other in a limited area for existence; this is the standard model of natural selection according to Darwin. It is clear that in this struggle, the most adapted organisms can survive, and it is they who occupy the dominant position in the daughter community. As a result of this displacement, the remaining species die due to oppression or inability to coexist, which leads to a sharp drop in biodiversity. Notably, the end point of the hump in Figure 2 is the steady state of the climax community, in which the surviving species coexist with each other and reach ecological equilibrium. In other words, the rate of successional processes differs significantly over time and undergoes ups and downs, which forms the hump shape of the daughter community’s biodiversity.
References
Boundless. (2021). Evolution of Angiosperms. LibreTexts. Web.
ICE Real. (n.d.). Why flowering plants are so diverse. Learn Genetics. Web.
Knelman, J. E., Graham, E. B., Prevéy, J. S., Robeson, M. S., Kelly, P., Hood, E., & Schmidt, S. K. (2018). Interspecific plant interactions reflected in soil bacterial community structure and nitrogen cycling in primary succession. Frontiers in Microbiology, 9, 128-140.
Rhoades, H. (2021). Cross pollination in plants: Cross pollinating vegetables. Gardening Know How. Web.