Abstract
The aim of this lab experiment was to study the effect of temperature and acidity (pH) on the solubility of carbon dioxide in water as it happens in oceans. The study was conducted in a laboratory setting. The results indicate that the solubility of carbon dioxide is significantly reduced by both increase in water temperature and acidity. These results have the implication of global warming and climate change and the associated environmental, economic, and social consequences.
Climate change is a global issue that is affecting every part of the world. Monitoring the progress of climate change and global warming is necessary to determine the extent to which human activities are affecting the environment (Jiang et al., 2019). This laboratory experiment was conducted to test the solubility of carbon dioxide in seawater. The solubility of carbon dioxide is directly related to climate change because oceans absorb excess carbon dioxide from the atmosphere (Doney et al., 2020). Nevertheless, due to excessive release of carbon dioxide into the atmosphere by human activities, the oceans are overwhelmed and cannot absorb as much as anticipated (Gao et al., 2019). Industries, farming activities, burning vegetation and plastic waste, burning petroleum products in transportation, and other human activities all produce excessive carbon dioxide into the atmosphere (Ali et al., 2019). This excessive carbon dioxide then acts as a heat-trapping greenhouse gas in the atmosphere (Gao et al., 2019). The objective was to simulate increasing atmospheric carbon dioxide levels as well as temperature and observe the effects on pH. The experiment sought to determine how both temperature and pH affect the solubility of carbon dioxide in water.
Study hypothesis
It was assumed that an increase in pH and temperature of the solution reduces solubility of carbon dioxide and therefore the ocean’s capacity to serve as the carbon source rather than carbon absorber. Furthermore, if the carbon dioxide concentrations continue to rise, coupled with increase in ocean temperatures, the ocean water will reach saturation and no longer able to absorb the excess carbon in the atmosphere, thus causing climate change.
Variables
The independent variables are the pH and temperature and will be manipulated to observe how they affect the solubility of carbon dioxide in water. In contrast, the solubility of carbon dioxide in water is the dependent variable, which will be observed and measured as the outcomes of the experiment.
Expected outcomes
It is expected that the solubility of carbon dioxide in water will reduce with an increase in both pH and temperatures.
Materials and Methods
In each of the groups, the materials provided for the experiment were:
- Brown tray (to catch spills and carry supplies)
- 250 ml graduated cylinder
- 400 ml beaker
- Funnel
- One half of a Petri dish
- Large plastic tub
- Thermometer
- 6 Effervescent tablets
Experiment procedure
The students were divided into 2 groups, each having between 4 members. The groups receive the same set of materials as indicated above.
- All the groups filled up the plastic tub 2/3 to ¾ full of water and ice. The water was mixed around until a consistent temperature of 8-10 oC was achieved.
- Using pH paper, the groups measured the pH of your water, which was recorded in Table 1.
- Then, they placed an inverted funnel at the center.
- The graduated cylinder was filled to the top with cold water from the tub, taking care not to get any ice cubes. Then, it was placed carefully upside down in the basin on top of the funnel.
The petri dish was used to cover the mouth of the cylinder while being inverted and placed in the container. After the cylinder was underwater, the petri dish was removed. taking cate to avoid water spills and formation of air bubbles.
- One group member held the cylinder in place.
- The water temperature was then recorded
- Another member placed an effervescent tablet under the funnel, making sure that the hands were dry when handling the tablet.
- After the tablet dissolved, the level of the air space that formed was observed. The investigation was repeated for a total of three times with the same conditions. The same water was used by refilling the cylinder full before each trial. The results were recorded in Table 1.
Table 1. Conditions and data for each trial.
An independent Each group was changed- some groups changed temperature while the others changed the pH of the water.
- Temperature groups –about half of the water was poured out and the temperature of the water was changed by adding hot water until the temperature was close to 20oC.
- pH groups – The pH was changed by adding 100 ml of vinegar.
- The conditions were recorded in Table 2.
- The temperature and/or pH were kept as consistent as possible for all three trials.
Table 2. Conditions and data for each trial.
Class data was recorded in Table 3.
Data was then entered in Excel and the mean and standard deviation for all three conditions were calculated.
Results
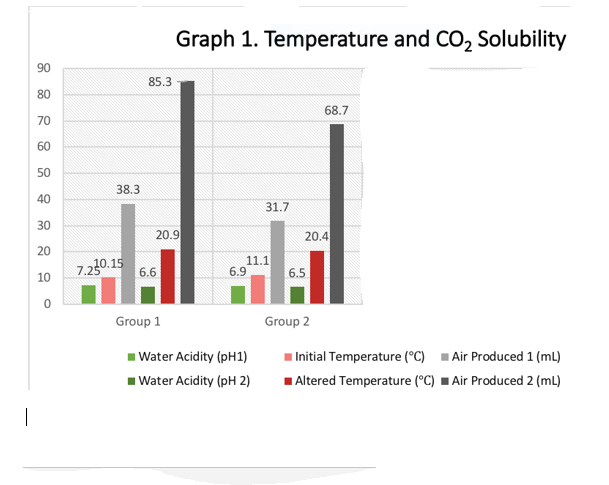
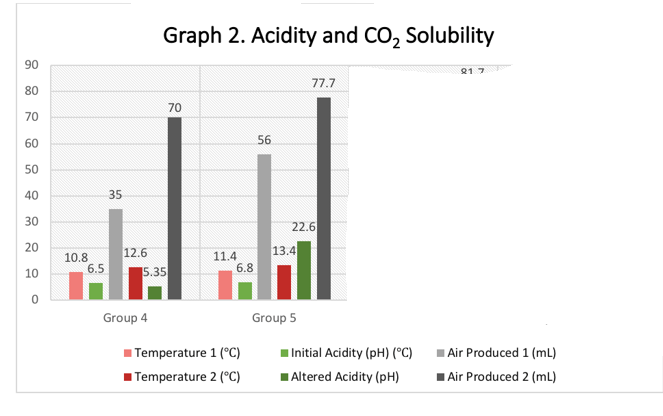
The data supported the hypothesis that increased temperatures causes a decrease in the solubility of carbon dioxide in water. Secondly, increased acidity causes a decrease in the solubility of carbon dioxide in water. Consequently, carbon dioxide is less absorbed by water that is warm and acidic. The class data shown in table 3 show these relationships as portrayed by the data
Table 3. Class data
Discussion
The results show an inverse relationship between temperature and carbon dioxide solubility and between acidity and carbon dioxide solubility. In essence, the solubility of carbon dioxide decreases with an increase in water temperature and acidity. In nature, the more the carbon dioxide is emitted into the atmosphere, the less the ability of ocean water to absorb excess emissions (Middelburg, Soetaert & Hagens, 2020). Carbon dioxide molecules form bonds with water molecules to form carbonic acid, which then dissociates to form hydrogen ions and bicarbonate. The hydrogen ions than form bonds with carbonate for self-neutralization. As a result, less carbonates are left in the ocean water for calcium binding to form calcium carbonate, which is necessary for the formation of shells in marine organisms. Therefore, there is a deficit of carbonate taken by carbon dioxide, which affects the organisms.
Nonetheless, excessive emission of carbon dioxide in the atmosphere implies that ocean waters try as much as possible to absorb it, but reach a point of saturation beyond which no more carbon molecules can dissolve. Atmospheric temperatures increase due to an elevation in the amount of carbon dioxide emitted from human activities (Agostini et al., 2018). Also, the acidity of water increases as more carbon enters the oceans. With time, both oceanic temperatures and acidity increase, which reduces the ability of the water to absorb carbon dioxide. Consequently, global warming and climate change occur, which affect humans through increase in the frequency and extent of such phenomenon as droughts, desertification, floods, storms, and temperatures (Hall-Spencer & Harvey, 2019). Social consequences include competition for the reducing resources such as arable land, fish, water, and others, which can also involve political and civil conflicts.
References
Agostini, S., Harvey, B. P., Wada, S., Kon, K., Milazzo, M., Inaba, K., & Hall-Spencer, J. M. (2018). Ocean acidification drives community shifts towards simplified non-calcified habitats in a subtropical− temperate transition zone. Scientific Reports, 8(1), 1-11.
Ali, M., Al-Anssari, S., Arif, M., Barifcani, A., Sarmadivaleh, M., Stalker, L., Lebedev, M., & Iglauer, S. (2019). Organic acid concentration thresholds for ageing of carbonate minerals: Implications for CO2 trapping/storage. Journal of Colloid and Interface Science, 534, 88-94.
Doney, S. C., Busch, D. S., Cooley, S. R., & Kroeker, K. J. (2020). The impacts of ocean acidification on marine ecosystems and reliant human communities. Annual Review of Environment and Resources, 45(1).
Gao, K., Beardall, J., Häder, D. P., Hall-Spencer, J. M., Gao, G., & Hutchins, D. A. (2019). Effects of ocean acidification on marine photosynthetic organisms under the concurrent influences of warming, UV radiation, and deoxygenation. Frontiers in Marine Science, 6, 322.
Hall-Spencer, J. M., & Harvey, B. P. (2019). Ocean acidification impacts on coastal ecosystem services due to habitat degradation. Emerging Topics in Life Sciences, 3(2), 197-206.
Jiang, L. Q., Carter, B. R., Feely, R. A., Lauvset, S. K., & Olsen, A. (2019). Surface ocean pH and buffer capacity: past, present and future. Scientific Reports, 9(1), 1-11.
Middelburg, J. J., Soetaert, K., & Hagens, M. (2020). Ocean alkalinity, buffering and biogeochemical processes. Reviews of Geophysics, 58(3), e2019RG000681.