Introduction
Photosynthesis is fundamental to the energy flow process in living organisms. “Plants are the primary producers and they make use of sunlight to produce sugars for energy production.” (Govindjee, 1997, p. 45) Excess nutrients are stored and the plants are eaten up by herbivores and omnivores which rely on the energy stored in the plant cells to keep alive. The herbivores are subsequently consumed by the omnivores and carnivores; this process continues from one living organism to another creating a food chain that sustains life on earth.
Problem statement
The energy flow process is fundamental to the sustenance of life on earth. For survival, each organism requires nutrition; the nutrition is sourced from another organism as food substrates except for the green plants which manufacture their food using sunlight and minerals. Many types of research have been conducted and reveal that the process of photosynthesis is the main process that sustains life. This experimental study is designed to ascertain how the process of photosynthesis leads to energy production and how it is affected by variation in light intensity and wavelength.
Relevance of the question
This study is essential for a proper understanding of the role of plants as primary producers in the food chain process.
Literature review
The photosynthesis process occurs primarily in the leaves with little taking place in the stem for some plants. The main parts of the leaf in which are involved in the process include; “the upper and lower epidermis, the mesophyll, the vascular bundles and the stomates.” (Photosynthesis, 2000) The epidermis lacks chlorophyll and therefore photosynthesis does not occur there, the epidermis only acts to protect the internal part of the leaf. “The stomates are tiny holes in the epidermis through which gaseous exchange takes place.” (Photosynthesis, 2000, para.2)
Through the stomates, Co2 enters, and O2 leaves. “The vascular bundles constitute the plants transport system through which water and other nutrients are moved around the plant.” (Photosynthesis, 2000, para.2)

Chlorophyll is composed of the outer and inner membranes, “there is also an inter membrane space stroma and thylakoids which are stacked in grana. The chlorophyll is normally built in the membrane of the thylakoids.” (Photosynthesis, 2000, para.3)
Thus, photosynthesis is the main process in which the “radiant light energy is absorbed by the chloroplast’s pigment, chlorophyll and converted into chemical energy in the molecular form of ATP and NADH.” (Photosynthesis, 2000) The energy is then utilized in driving the Calvin cycle in the production of three-carbon sugars. The three-carbon sugars are subsequently converted to various types of carbohydrates.
According to Govindjee, it is currently possible to carry out photolysis in the laboratory. The reaction was first performed by Robert Hill in 1937 and thus became known as the Hill Reaction. The Hill Reaction requires only intact isolated chloroplasts rather than the entire intact plant cell. However, since isolated chloroplasts do not reduce carbon dioxide directly, it is essential to provide a hydrogen acceptor for the reduction process and permit electron transport to take place. The hydrogen acceptor that was chosen for this experiment is the synthetic compound 2,6-dichlorophenol-indophenol or DPIP, which is blue in the oxidized form and colorless when reduced. As indicated above, the source of hydrogen for the reduction in water. This hydrogen can reduce DPIP and turn it from a blue substance to a colorless one.
Oxygen is also formed, but it is not monitored in this experiment. The change in color of the DPIP solution, which is directly proportional to the number of hydrogen produced in the Hill Reaction, can be assayed using colorimetric spectrophotometry. The more hydrogen produced, the more DPIP that is reduced and the more colorless the solution containing DPIP becomes. Therefore, the course of this reaction can be assessed by the bleaching of the artificial hydrogen acceptor. (1997, p. 144))
Light + CO2 + 2H2O → n (CH2O) + H2O + O2
The experiment entailed the use of both boiled and fresh chloroplast suspension. The boiled chloroplast was used as a control and photosynthetic activities were monitored in the fresh chloroplast. The results were measured using a spectrophotometer and tabulated. The same procedure was repeated with variations in the light intensity and wavelength.
Materials
- Spectrophotometer
- Spectrophotometer cuvettes
- Phosphate buffer (pH6.5)
- Chloroplast suspension
- Sodium hydrosulfite
- Distilled water
- Wrapping foil
- Digital timer
- DPIP solution
Procedure
The source of hydrogen for the reduction in water. This hydrogen can reduce DPIP and turn it from a blue substance to a colorless one. Oxygen is also formed, but it is not monitored in this experiment. The change in color of the DPIP solution, which is directly proportional to the number of hydrogen produced in the Hill Reaction, can be assayed using colorimetric spectrophotometry. The more hydrogen produced, the more DPIP that is reduced and the more colorless the solution containing DPIP becomes. Therefore, the course of this reaction can be assessed by the bleaching of the artificial hydrogen acceptor. The experimental procedure was selected because it has a high specificity and therefore results will have a low error margin. The chemicals used are readily available were purchased in various forms and reconstituted in the laboratory before the practice according to the manufacture’s guidelines.
Procedure
- Obtain fresh and boiled chloroplast suspension prepared before the practical time. The boiling of the suspension will render the chloroplasts non-functional and will serve as one of the experimental controls. Allow the solution to return to room temperature before use. The chloroplast is boiled preferably at 100 degrees for 5 to 7 minutes.
- Obtain four clean spectrophotometer cuvettes. Label them 1-4 and prepare them as follows: Cuvette 1: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of boiled chloroplast suspension, Cuvette 2: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of chloroplast suspension, Cuvette 3: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of chloroplast suspension, Cuvette 4: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of chloroplast suspension
- Add 50 μl of the 0.1% DPIP solution to cuvette 4 only. Shake it to mix the solution well. Then add a few (very few) crystals of sodium hydrosulfite (Na2S2O4) to it. Sodium hydrosulfite reduces the DPIP and removes the blue color.
- Be sure that the spectrophotometer is set to 600nm. Use the ‘reduced’ cuvette 4 as a blank to zero the spectrophotometer.
- Add the same amount of DPIP that you added to cuvette 4 to cuvette 1. Mix the solution well and take a reading as quickly as possible using the spectrophotometer, because cuvettes with functional chloroplasts should immediately start producing hydrogen that reduces the DPIP. Record this value as ‘time zero’. Start the digital timer.
- Repeat step 5 for cuvettes 2 and 3. Record the ‘time zero’ values and their starting time points.
- Immediately after taking the first readings, wrap cuvette 3 completely in aluminum foil and place cuvettes 1-3 in a beaker of water at room temperature. Set the light source 10 inches from the cuvettes and turn it on to the highest setting. Note that the water in the beaker serves as a thermal buffer to prevent any experimental artifact due to warming by the source light.
- Every two minutes record the optical density of cuvettes 1 and 2. Leave cuvette 3 in the dark until the end of the procedure.
- Continue taking readings until the optical density reading of cuvette 2 does not change.
- Remove cuvette 3 from its foil wrapping. Quickly read the optical density of this cuvette. Take two-minute readings until the optical density reading does not change for two or three-time points. Make a graphical plot with time as the independent variable and optical density as the dependent variable.
Note: Different colors of light indicate the difference in wavelength thus the use of various colors of cellophane to measure wavelength.
Dependent, independent, and controlled variables
The independent variable is the time of exposure whereby the duration of the reaction is changed to determine the effect of time on the DPIP reduction (visualized as the clearing of the blue color). The dependent variable is the optical density which varies depending on the duration the cuvette was left to react. The controlled variables include the use of the same amount of constituent chemical for each tube, temperature of the reactions where all the tubes are reacted at room temperature.
Threat reduction to internal validity was done by:
Taking measurements immediately after the timed duration had expired; was done to prevent errors in the measurement of the optical density. Providing the same conditions for all measurements taken i.e. the spectrophotometer was blanked by the same solution for all the readings which was done at 600nm. All the necessary conditions for the materials were provided prior to and during the experiment.
Hypothesis
Plants form an important part of the food chain. “Green plants manufacture their on food using the photosynthesis process.” (Photosynthesis, 2000, para.1) Sunlight is an important aspect of this process; the practical examines the process of photosynthesis, particularly the role played by light. Therefore:
HA: Light is a fundamental factor for the photosynthetic process where it’s used to reduce carbon dioxide and break the water molecule. In this experiment, the hydrogen from the water molecule reacts with DCIP to reduce its blue color. The spectrophotometer is used to measure the intensity of the reduced color.
Ho: Light is not a fundamental factor in the process of photosynthesis and does not reduce carbon dioxide or break the water molecule to produce hydrogen used in the experiment to reduce DCIP’s blue color that is measured using a spectrophotometer.
Use of appropriate methods, tools, and technologies to collect quantitative data
In this experiment quantitative data was collected as optical density readings. The readings were taken at 600 nm after the spectrophotometer was blanked using cuvette 4 which had 3.0 ml of phosphate buffer (pH 6.5) 1.0 ml of chloroplast suspension and 50 μl of the 0.1% DPIP solution added and the reaction left to complete( color clears). The optical density reading for each cuvette was recorded against the time duration in the lab notebook.
Data Collection
Data collection is an important aspect for the success of any experiment, for this particular experiment, the data was collected by the use of a spectrophotometer. Every reaction was timed according to the manual and on expiry of the time duration, the optical density readings were taken using the spectrophotometer and recorded in the laboratory notebook for use in the tabulation and plotting of graphs for analysis.
Experiment: the hill reaction.
Results of the Experiment
The results for the experimental were plotted as below
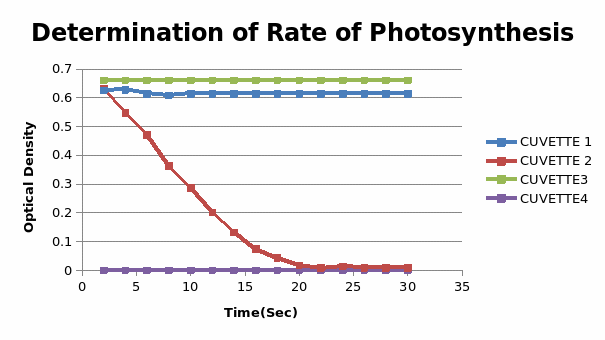
This experiment was designed to investigate the photosynthesis process in which light is utilized for energy production. In the hill reaction, the rate of photosynthesis remained the same for cuvettes 1 and 3, there was no evidence of photosynthetic activity in the fourth cuvette. In cuvette 5, the photosynthetic activity decreased with time. From the experiments, it is deduced that photosynthesis occurs in two stages, the light-dependent, and the light-independent stage. Generally, “in the first stage energy is captured and stored in the form of ATP and NADPH.” (Photosynthesis, 2000)
In the second stage light-independent stage the energy stored is used to process sugars using carbon. The results of this experiment agree with the hypothesis that light is a fundamental factor for the photosynthetic process where it’s used to reduce carbon dioxide and break the water molecule. In this experiment, the hydrogen from the water molecule reacts with DCIP to reduce its blue color. The spectrophotometer is used to measure the intensity of the reduced color
Conclusion
The experiment was carried out successfully and the results indicate that indeed plants manufacture their own food using sunlight as a source of energy. Therefore sunlight is an important factor in this process. However, the success of the practical was due to the experimental design which provided all the necessary material and conditions for the laboratory model of photosynthesis. A good experimental design gives results with minimal errors and thus can be used to conclude. “Validity refers how closeness the values are for repeated measures.”(Govindjee, 1997, p. 67) Replication of the above experiment gives results from which comparison can be drawn to give a test of validity for this experimental design.
The experiment can be replicated by another person provided he/she formulates a suitable experimental design that will include the identification, manipulation, and measurement of all the parameters in the investigation. The experimental design must be reliable and carefully selected to reduce the error margin to minimum values as this is an important aspect of this experiment.
Reference list
Govindjee, G. (1997). Experiments in plant biology. Berlin: Springer.
Photosynthesis. (2000). Web.