Introduction
In the contemporary world driven by progress in science and technologies, the strive toward sustainable and ecological agricultural methods prevails. Vastly utilized chemical fertilization provides a significant yield increase as it acts as a growth stimulant for plants. However, agricultural companies tend to use chemicals in excess, which has a cumulative adverse influence on the land. Chemical fertilizers are not environment-friendly, as they act as pollutants and are harmful to the soil in a long-term perspective. Indeed, according to Suhag (2016), abundant utilization of chemical fertilizers is not cost-effective and causes significant pollution of ecosystems.
Therefore, there is a persistent need for natural and sustainable methods that would increase plant growth and health without causing disruptive damage to the soil and the ecosystems in general. For that matter, extensive and precise research is necessary to identify and test some of the most effective, cost-efficient, and sustainable methods of improved plant growth development.
In particular, the growth and quality of legumes are important for the whole sphere of agriculture. Firstly, legumes are a fundamental crop constituent used as a valuable food source globally (Stagnari et al., 2017). In addition, these plants yield sustainable agriculture since they allow for minimizing greenhouse gas emissions, as they produce approximately 5–7 times less greenhouse gas than other agricultural plants (Stagnari et al., 2017). Thanks to their nitrogen fixation ability, legumes “fix the atmospheric nitrogen, release in the soil high-quality organic matter and facilitate soil nutrients’ circulation and water retention” (Stagnari et al., 2017, p. 1). Thus, it is essential to investigate the improvement of the yield and feed quality of legumes to ensure sustainable agriculture.
The investigation of rhizobia strain in legumes is of particular relevance to the study goals because rhizobia are specifically active in nitrogen fixation in legume root nodules. According to Peralta et al. (2016), rhizobia establish nitrogen-fixing symbioses with legumes, which stimulate the formation of nodules on the legume roots. Due to rhizobia nitrogen fixation, atmospheric dinitrogen is reduced into ammonium, which is ultimately exported to the plant (Peralta et al., 2016).
This process is important because it increases legume growth, improves ecosystems’ development, and changes diammonium into a usable nitrogen form for plants (Gano‐Cohen et al., 2019). Moreover, when using legumes that fix N, it is possible to reduce chemical N-fertiliser use. Thus, investigating the difference in the growth of legumes with and without right or wrong rhizobia is required to provide evidence on sustainable fertilization.
The current study is designed to identify how nodulation is impacted by rhizobia and if nodulation impacts plant growth and health. The aims of the study include:
- identify and measure the difference between non-ideal, ideal inoculant, and no inoculant-induced growth changes;
- find evidence-based proof of positive correlation between rhizobia inoculation and plant growth and nodulation;
- generate new scientific findings and contribute them to the modern-day agricultural sphere for more sustainable method application.
The current study addresses the identified gap in the academic literature related to the lack of practically tested difference between inoculation using right rhizobia type, wrong rhizobia type, and no rhizobia treatment. The novelty of the study findings will be applicable to specialists practicing plant growth in the agricultural domain for safe and cost-efficient harvest increasing. Moreover, the findings might have implications for researchers in agriculture and sustainability since they provide a basis for further investigation of plant growth and health under the influence of additional factors.
Materials and Methods
For the purposes of the study, the method of the experiment was used to measure the growth indicators in three treatments of clover plants. The method of experimentation is regarded as one of the most rigorous methods in research that is capable of producing reliable and objective data (Rosenbaum, 2018). Overall, 63 plants were used for the study; they were grouped into three treatment categories. The categories were as follows: three plants that had been exposed to the correct rhizobia treatment, three plants that had been exposed to the wrong rhizobia treatment, and three plants that had not been exposed to any treatment. Seven different groups of researchers investigated a set of nine plants in total.
Overall, seven measurements were conducted for each of the plant categories in a one-plant-at-a-time manner. The measurements included nodule number, nodule score, root length, shoot height, root fresh mass, shoot fresh mass, and Soil-Plant Analyses Development (SPAD) value. While the physical characteristics such as length, height, mass, and nodule number were measured using a ruler and weights and the results were observed without specific devices, SPAD was conducted using a SPAD meter. SPAD values indicate the greenness of plant leaves, showing the amount of chlorophyll in the plants that are needed for photosynthesis (Borhan et al., 2017). Thus, the measurements were aimed at retrieving data pertaining to both the growth and health of the plants.
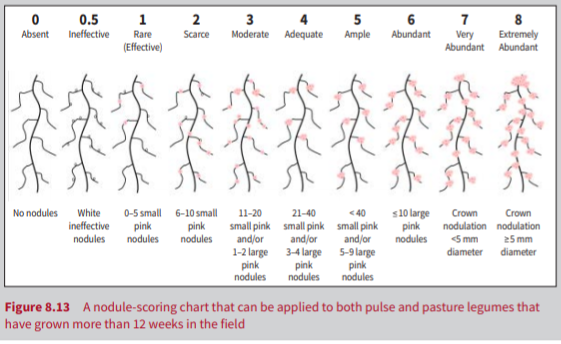
The experiment was conducted by seven groups of researchers separately to ensure an in-depth investigation of rhizobia treatment’s impact on nodule number, shoot height and mass, root length and mass, nodule score, and SPAD values. Overall, 63 plants were used for the experiment, with nine plants per one research group. First, the plants were extracted from the soil, and the roots were cleared from any soil and dried with a paper towel. The nodules were carefully removed from the roots and calculated. Nodules were then cut in half using a scalpel to see the color of the inside. The nodule score was identified through the comparison of their density, size, and color to the control table (Fig. 1).
After that, to measure shoot and root height/length and mass, the roots were separated from shoots, and necessary measurements were made using a ruler applied to the longest strands of root/shoot of the plant. The weight of each root and shoot was measured using the scales. Finally, the SPAD value of the plant leaves was measured using a SPAD meter; three consecutive measurements were conducted to identify a mean value for each plant.
All data were recorded and placed into an excel table with respective columns representing each measurement and rows representing replications. After the experiment was finished and all necessary data collected, the numbers were analyzed and the findings compared. The results of all groups’ experimentation were taken into consideration to ensure a large number of replications. The results of the study are presented in the next section of this report.
Results
The results of the experiment showed a difference between the control plants and those exposed to either wrong rhizobia or correct rhizobia treatment. The study findings are presented in Table 1. Non-ideal stands for plants exposed to treatment using the wrong rhizobia, ideal inoculant stands for plants treated with the correct rhizobia, and control stands for plants that were not treated. The control plants are used for comparison with those treated with correct and wrong bacteria.
Table 1. Experiment Results: Comparative Table of Legume Growth Indicators in Plants Exposed to Non-Ideal Inoculant, Ideal Inoculant, and Control.
The results show that nodulation was improved in replications that were exposed to treatment using the correct rhizobia. Both the number of nodules and their score were significantly higher in plants after correct bacteria treatment in comparison to control plants and non-ideal inoculant plants. Importantly, the number of nodules and their score of non-ideal inoculant plants were lower than those of the control group, which implies that no treatment yields better results than treatment with wrong bacteria.
Apart from nodulation changes after treatment, the effect of nodulation on plant growth and health was tested. The results of all groups indicate that nodulation had a negative impact on plant shoot length, which was lower in plants of ideal inoculant than in those of control and non-ideal categories. However, the root lengths did not differ significantly across all plant categories. Importantly, shoot fresh mass and root fresh mass of plants exposed to the correct rhizobia treatment were higher in comparison to those treated with the wrong bacteria and was on average higher than the mass of roots and shoots of plants from the control category.
As for the SPAD analysis results, they revealed that the greenness of leaves of plants after the correct rhizobia treatment was higher in value than that of the other two categories of plants. Overall, the results demonstrate that clover plants exposed to the correct rhizobia treatment have a bigger number of root nodules with higher nodulation scores, higher SPAD value, and root and shoot fresh mass in comparison to non-ideal and control categories. However, shoot height is lower, and root length does not differ significantly from the two other categories of plants. The implications of these findings are further discussed in the next section of the report.
Discussion
The general study results are compatible with the overall research context around the investigated problem. The increase in nodulation caused by rhizobia that has been found in this study coincides with the findings presented by Sepulveda-Caamano et al. (2018), who proved in their experimental study that rhizobia increase the nodulation of lentils. Similarly, the findings of the present study suggest that using a correct type of bacteria for treatment yields better nodulation results are consistent with the findings of Argaw and Mnalku (2017).
They found that the use of the correct (or native, as the scholars call them) rhizobia “inoculation resulted in the highest nodulation and grain yield production as compared to the other treatments” (Argaw and Mnalku, 2017, p. 1390). However, this study contributes its novelty to the body of academic literature on the topic as it provides accurate data on such measurements as root and shoot mass, SPAD values, and nodulation score.
Moreover, the findings of this study provide a practical implication for solving problems with plant nodulation identified in academic literature. Indeed, according to a recent study conducted by Hackney et al. (2017), the decreased nodulation in the pasture is risky in terms of pasture quality and sustainability of agriculture overall under the influence of plant growth inconsistency. The researchers found that since inadequate nodulation was observed in “more than 90% of the 225 pasture paddocks,” it is “unlikely that pasture legumes are fixing nitrogen in quantities suggested in the literature” (Hackney et al., 2017, p. 3).
Therefore, the improvement of nodulation under the influence of the correct rhizobia treatment found in this study will be helpful in eliminating the problem identified by Hackney et al. (2017). In addition, the findings add to the argument made by Xie et al. (2020), who proved that soil bacteria are capable of significant improvement of plant productivity and overall better sustainable capacity of agriculture. Thus, plant growers might apply the findings of this study by using rhizobia treatment for increasing plant growth, health, and yield.
The findings of the current study showed an increased value of SPAD in the correct rhizobia-treated plants. It implies that these plants fix nitrogen better as demonstrated by Xie et al. (2020). Moreover, as has been suggested by Kanomanyanga et al. (2021),” the ability of symbiotic rhizobial bacteria to fix atmospheric nitrogen in legume plants can improve grain yield without applying nitrogen fertilizer” (p. 207). Thus, consistent with the academic literature, the present study provides a solid ground for implementing the correct rhizobia treatment for increased nodulation, better plant health, and growth, as well as improved nitrogen fixation for better environmental outcomes.
Conclusion
In summation, this report has presented the aim, procedure, results, and academic and practical implication of the research on the effect of rhizobia strain on nodulation and the impact of nodulation on plant growth and health. The study found that the treatment of legumes with the correct rhizobia helps to increase the number of root nodules in the plants compared to the plants treated with the wrong rhizobia and the plants without treatment. Moreover, the nodulation score of the correctly treated plants was higher than that of the other two categories of plants, as well as root length and fresh mass, and shoot fresh mass were increased. It was also found that the plants treated with the correct type of bacteria are healthier and have a higher SPAD value in comparison to no-treatment and wrong bacteria treatment.
While these results are consistent with the general academic findings concerning native bacteria treatment effectiveness and increased nitrogen fixation of legumes with more nodules, the study contributes a novelty to the academic field. It provides a detailed overview of nodulation, growth, and health outcomes and allows for the evidence-based implementation of the correct rhizobia treatment to yield a better pasture harvest and improve agricultural sustainability.
Reference List
Argaw, A. and Mnalku, A. (2017) ‘Effectiveness of native Rhizobium on nodulation and yield of faba bean (Vicia faba L.) in Eastern Ethiopia’, Archives of Agronomy and Soil Science, 63(10), pp. 1390-1403.
Borhan, M.S. et al. (2017) ‘Evaluation of computer imaging technique for predicting the SPAD readings in potato leaves’, Information Processing in Agriculture, 4(4), pp. 275-282.
Gano‐Cohen, K.A. et al. (2019) ‘Interspecific conflict and the evolution of ineffective rhizobia’, Ecology Letters, 22(6), pp. 914-924.
Hackney, B. et al. (2017) ‘Nodules or not–a survey of pasture legume nodulation in central and southern NSW’, Proceedings of the 18th Australian Society of Agronomy Conference. Web.
Howieson, J. G. and Dilworth, M. J. (eds.) (2016) Working with rhizobia. Canberra: Australian Centre for International Agricultural Research.
Kanomanyanga, J. et al. (2021) ‘Meta-analysis of the effects of Rhizobia inoculants and phosphorus fertilizer on soybean nodulation in Africa’, African Journal of Plant Science, 15(8), pp. 206-224.
Peralta, H. et al. (2016) ‘Genomic studies of nitrogen-fixing rhizobial strains from Phaseolus vulgaris seeds and nodules’, BMC Genomics, 17(1), pp. 1-18.
Rosenbaum, P. (2018) Observation and experiment. Cambridge: Harvard University Press.
Sepulveda-Caamano, M. et al. (2018) ‘Lentil (Lens culinaris L.) growth promoting rhizobacteria and their effect on nodulation in coinoculation with rhizobia’, Archives of Agronomy and Soil Science, 64(2), pp. 244-256.
Stagnari, F. et al. (2017) ‘Multiple benefits of legumes for agriculture sustainability: an overview’, Chemical and Biological Technologies in Agriculture, 4(1), pp. 1-13.
Suhag, M. (2016) ‘Potential of biofertilizers to replace chemical fertilizers’, International Advanced Research Journal in Science, Engineering and Technology, 3(5), pp. 163-167.
Xie, M.M. et al. (2020) ‘Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover’, Plant, Soil and Environment, 66(6), pp. 287-294.