Introduction
The study of the physicochemical properties of substances in the context of the intermolecular forces that are realized for each of the compounds is an essential part of the analytical study. In general, intermolecular interactions depend on the nature of the substance, namely the presence of a hydroxyl group and the polarity of the molecule. Since it is known that hydrogen bonds are formed between atoms of different compounds with a high difference in electronegativity, such binding is characterized by high strength, in contrast, for example, to Van der Waals forces. A higher binding energy requires the application of more energy, including thermal energy, to break bonds between molecules. Such breaking makes sense during phase transitions of substances, including when a liquid turns into a gas, which means that the distance between molecules increases rapidly and the intensity of intermolecular bonding drops. To quantify the described transition, there is the Clausius-Clapeyron equation, shown in the formula [1].

In this equation, P is the vapor pressure, which is actually the pressure that vapor exerts when it is in equilibrium with its liquid form. ∆Hvap is the enthalpy (heat) of vaporization, which corresponds to the amount of thermal energy required to convert one mole of a substance to a gaseous state from its liquid form. Finally, R and T are the standard thermodynamic parameters: the gas constant (8.314 J.mol-1.K-1) and temperature. The equation predicts that a study of the pressure-temperature relationship for a particular substance during heating can be used to calculate the enthalpy of vaporization. In turn, such a process can be used to qualitatively define a compound, determine its chemical purity, or calculate the optimal chemical-technological conditions for the substance. The calculation of the enthalpy of vaporization can be performed by determining the slope of the graph of the original function, as shown in equation [2].
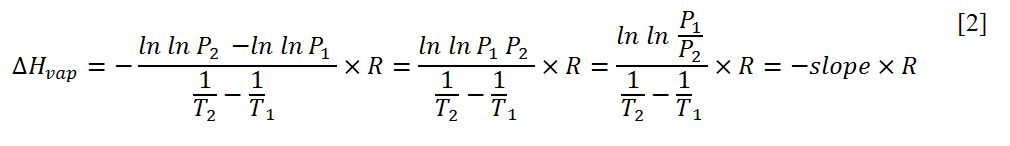
In the present experiment, these theoretical assumptions are used to determine the heat of vaporization for two substances, namely dichloromethane and ethanol. The substances differ in the presence of functional groups and polarity, and it can be predicted that, due to the presence of hydrogen bonds in the ethanol composition, the substance will require more thermal energy to break the intermolecular interactions. In other words, the primary working hypothesis is that the enthalpy of vaporization for ethanol will be higher than for dichloromethane.
Results
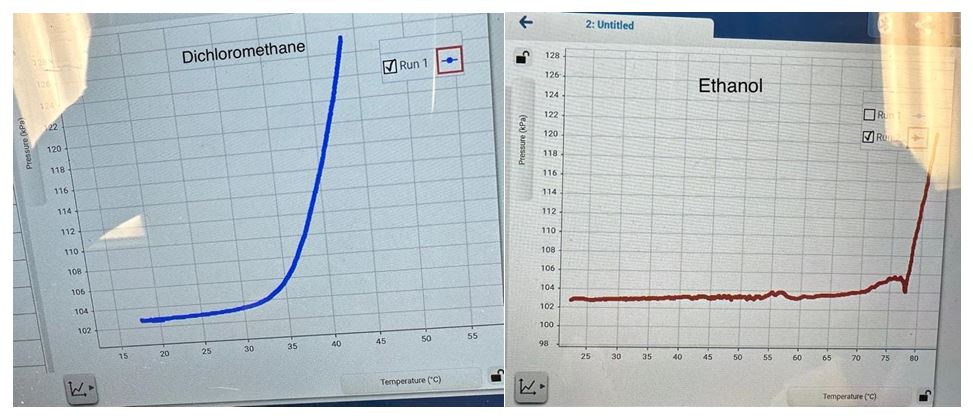
Table 1. Results of direct measurements of temperature and pressure for two substances, as well as the results of indirect calculations needed to create a scatter plot and regression straight line
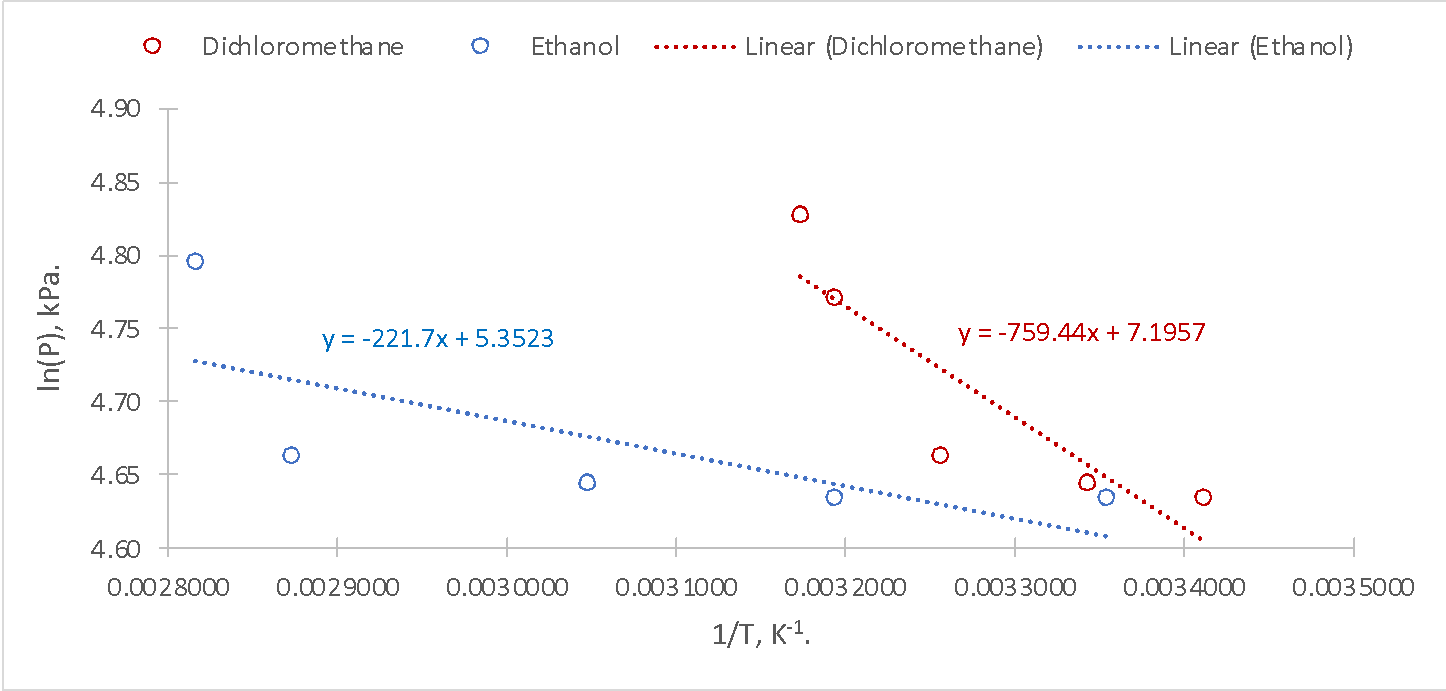
Fig. 3 made it possible to determine the slopes for the two dependences and therefore, the corresponding values of heat of vaporization can be calculated: equations [3]-[4] show these calculations.
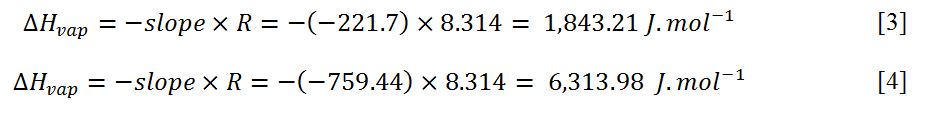
Conclusion
The purpose of this laboratory work was to determine the enthalpy of vaporization for two substances, dichloromethane and ethanol. The different natures of the substances, the presence of functional groups, and the difference in polarities were preconditions for the formation of the assumption that ethanol, which had hydrogen bonds, should have a higher heat of vaporization compared to dichloromethane, which was a polar molecule and had no hydrogen bonds. The measurement results and the regression equations, however, refuted this hypothesis: the heat of vaporization of dichloromethane was about 3.5 times higher for dichloromethane than for ethanol. This seems to be a contradictory result since the hydrogen bonds should be stronger than the Van der Waals forces, and therefore, the corresponding value of heat energy for the phase transition of one mole of ethanol should have been higher. Nevertheless, higher temperatures were shown for ethanol, leading to higher pressure compared to dichloromethane.