Introduction
Partition coefficient between marine water and passive samples of diuron, decadienal, atrazine, fluoranthene, and desethylatrazine compounds will be performed by spiking with a mixture and passive samplers are exposed. Standard compounds will be analyzed on the studying of the different time points when water and passive samplers are extracted and concentrated extracts are analyzed with HPLC-UV or HLPC-MS. The time that is needed for reaching the equilibrium is needed for the proper defining of the partition coefficient.
Partition coefficient
Diuron, decadienal, atrazine, fluoranthene, and deethylatrazine compounds are studied by numerous researchers, and definition of the partition coefficient is based on the necessity to identify the concentration of un-ionized compounds.
Methodology
Marine water samples are spiked at 1 mg/L. 50ml of water is spiked and ½ silicone sheet or 50 mg Sepra ZT is added. Sepra ZT is exposed for t=0, 1, 3, 7, 14 and 28 days, silicone sheets are exposed for t=0, 1, 5, 14, 28, 42 days. After extraction extracts are evaporated to 0.5ml and injected on a HPLC system and concentrations are determined with UV or MS. As a performance reference compounds several CB are used in the silicone rubber sheets and will be detected with GC-MS. Atrazinedesisopropyl-d5 is used as a reference compound in Sepra ZT.
Data Analysis
The data achieve may be analyzed in accordance with the relations found. The values of molecules in the solutions are associated with the observed differences of the volumes. The structural differences within the compound glass is studied by observing the effect on log D on diuron and decadienal atoms in PCB congeners (Rusina and Smedes1805). The positions of the atoms, as well as the partitioning coefficient was calculated in accordance with the following formulas:
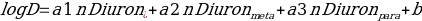
and
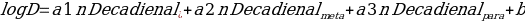
Where the intercept b is regarded as a log D of the molecule volume.
The components that are analyzed have the following partitioning coefficients:
The measured values are regarded as the decreased as the increasing partition sampling coefficient is lower in comparison with the hydrodynamic condition Rs ≈ Kpw-0.08.
As for the second part of the research, the rubber covers are used for proper dosing, and Rusina and Smedes (363) emphasized the following:
An excess number of spiked silicone rubber (SR) sheets was used for dosing the water phase. The uptake rate of target compounds by non-spiked SR passive samplers exposed in the same chamber was used to estimate the Rs over large hydrophobic range. The concentration in the dosing sheets is equal to the equilibrium concentration in the uptake samplers, while Cw in water can be calculated from the passive sampler-water partition coefficients (Kpw) and data interpretation is not hampered by uncertainty in measurement of Cw.
In the light of this fact, the time that is needed for reaching the equilibrium will depend on the parts of the solutions taken, and the values will be close to the following:
Conclusion
The partitioning coefficient of the water solutions with of diuron, decadienal, atrazine, fluoranthene, and desethylatrazine compounds are calculated in accordance with the formula based on defining the positioning of the atoms. The time needed for reaching an equilibrium in solutions calculated on the basis of the coefficients, and spiking of the SR passive samplers.
References
Rusina, Tatsiana, Foppe Smedes. Diffusion Coefficients of Polychlorinated Biphenyls and Polycyclic Aromatic Hydrocarbons in Polydimethylsiloxane and Low-Density Polyethylene Polymers. Research Centre for Environmental Chemistry and Ecotoxicology (RECETOX), Masaryk University, Kamenice 126/3, 625 00 Brno, Czech Republic. 2009
Rusina, Tatsiana, Foppe Smedes. Calibration of Silicone Rubber Passive Samplers: Experimental and Modeled Relations between Sampling Rate and Compound Properties. Environmental Science & Technology. 2010, 44, 362–367